October 6, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
Artificial Heart
This channel includes news and new technology innovations for total artificial hearts (TAH). These technologies are used in cardiovascular surgery to temporarily or permanently replace the heart in advanced heart failure patients.
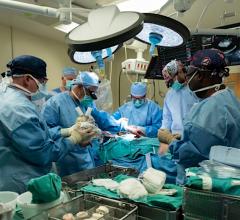
September 22, 2021 — A cardiothoracic surgical team with University of Louisville Health – Jewish Hospital has performed ...
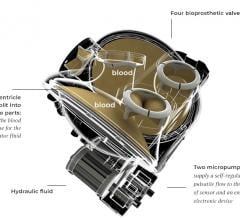
June 18, 2021 – An experimental artificial heart includes an autoregulation control mechanism, or Auto-Mode, that can ...
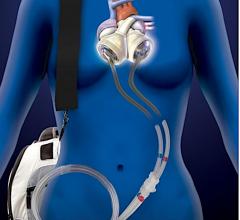
February 10, 2021 - The U.S. Food and Drug Administration (FDA) has granted artificial heart developer Carmat approval ...
December 2, 2020 — The Centers for Medicare and Medicaid Services (CMS) finalized updates to Medicare coverage policies ...
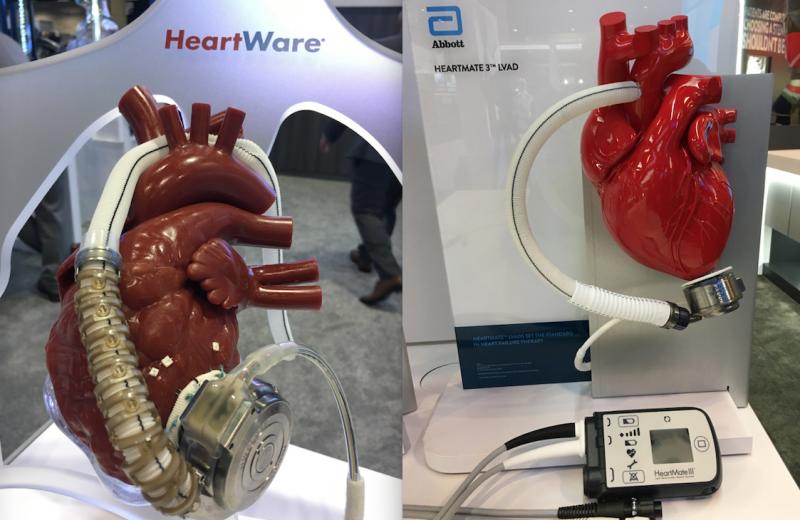
August 21, 2020 — The U.S. Centers for Medicare and Medicaid Services (CMS) proposed updates to coverage policies for to ...
May 27, 2020 — Carmat, a developer of the of a next generation advanced total artificial heart, announces the first ...
March 10, 2020 — The U.S. Food and Drug Administration (FDA) has cleared the SynCardia Systems 50cc temporary Total ...
March 5, 2020 — Abbott recently received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA ...
February 12, 2020 — The U.S. Food and Drug Administration (FDA) has approved Carmat's investigational device exemption ...
May 13, 2019 — A randomized clinical trial effectively used nerve stimulation through an ear clip to reduce atrial ...
December 11, 2018 — The scientific journal Nature recently published an article from Munich University Hospital which ...

October 19, 2018 — Abbott announced today that the HeartMate 3 Left Ventricular Assist Device (LVAD) has received U.S ...
January 25, 2018 – According to the latest market study released by Technavio, the global circulatory support devices ...
December 11, 2017 — CorInnova Inc. recently announced it was awarded second prize in the “2017 InnoSTARS” life science ...

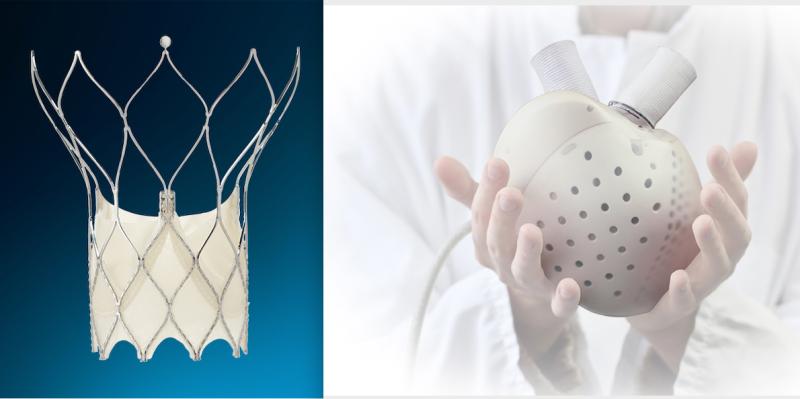
 October 06, 2021
October 06, 2021