Two of the top technology headlines this past month was the FDA clearance of the Abbott Portico transcatheter aortic valve replacement (TAVR) device, and the first women to receive a Carmat artificial heart.
October 6, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) magazine website from the month of September 2021. This is based on the website’s 200,902 pageviews for the month.
1. FDA Clears Fully Automated Cardiac Ultrasound Solution to Measure 2D and Doppler
2. First Woman in World Receives New Type of Artificial Heart
3. FDA Concerned About Higher Rates of LAA Occlusion Complications in Women
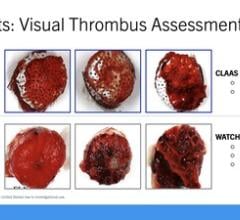
4. Abbott Amplatzer Amulet LAA Occluder Offers Superior Closure Compared to Watchman
5. TOMAHAWK Trial Does Not Support Early Coronary Angiography in Cardiac Arrest Without ST-elevation
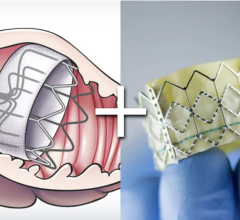
6. FDA Clears Abbott Portico TAVR System to Treat Aortic Valve Disease
7. MedAxiom Survey Highlights Cardiac Rehab Industry Insights and Best Practices
8. Continuous Heart Rhythm Monitoring and Anticoagulation Does Not Prevent Stroke
9. The Growing Need for Reprocessing in the Cardiac Cath Lab
10. FDA Clears Zebra Coronary Artery Calcium Solution as Part of Its Population Health Offering
Related Content:


 April 04, 2024
April 04, 2024