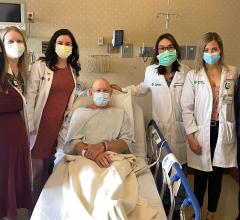
A patient who received the HeartMate III LVAD system showing off his external battery pack. He served as a patient ambassador in the Abbott booth at ACC 2018. The HeartMate III, with its magnetic levitated pump, showed a big reduction in clotting over previous LVADs in a key late-breaking trial at ACC earlier this year.
October 19, 2018 — Abbott announced today that the HeartMate 3 Left Ventricular Assist Device (LVAD) has received U.S. Food and Drug Administration (FDA) clearance as a destination therapy for people living with advanced heart failure. With the approval, physicians can now offer the HeartMate 3 system to patients not eligible for a transplant who will live with their device for the rest of their lives.
More than 5.7 million Americans suffer from heart failure, and there are approximately 915,000 new patients diagnosed with the disease annually. For advanced heart failure patients who can no longer rely on earlier stage treatment options, LVAD's take the workload off a weakened heart by pumping blood through the body—providing crucial, life-saving support for patients awaiting a heart transplant or for those not able to receive one.
"Approximately a quarter of a million people are living with advanced heart failure, and many of these people will need a heart transplant; however, only a few thousand will receive a new heart," said Nir Uriel, M.D., director of heart failure, transplant and mechanical circulatory support at the University of Chicago Medicine. "The destination therapy approval for Abbott's HeartMate 3 device now gives these patients new hope that they can receive a heart pump clinically proven to mitigate challenges we've historically confronted with this therapy — stroke and blood clotting — while also offering survival rates on par with transplant."
The HeartMate 3 pump utilizes technology known as Full MagLev (fully magnetically-levitated) Flow, which reduces trauma to the blood passing through the pump while improving flow.
"We partner with physicians to holistically develop therapies that benefit patients and achieve better outcomes," said Mike Pederson, senior vice president of Abbott's cardiac arrhythmias and heart failure business. "The unique design of the HeartMate 3 LVAD — with its Full MagLev technology — takes an established innovation and improves upon it in meaningful ways to help people with advanced heart failure live fuller lives."
The HeartMate 3 system's U.S. approval was supported by clinical data from the MOMENTUM 3 trial.[1] During the study, patients with the HeartMate 3 LVAD had an unprecedented survival rate of 82.8 percent at two years. Furthermore, rates of suspected pump thrombosis (clotting of blood) remained very low at 1.1 percent at two years. The study also showcased the lowest-ever published stroke rate (10 percent) for a continuous-flow LVAD at two years.
The MOMENTUM 3 study included more than 1,000 patients with New York Heart Association (NYHA) Class IIIB or IV heart failure. Patients were followed for a short-term endpoint of six months and a long-term endpoint of two years. Two-year data on the first 366 patients enrolled into the study were presented at the American College of Cardiology's (ACC) 67th Annual Scientific Session in March 2018 and simultaneously published in the New England Journal of Medicine.
The HeartMate 3 system includes the LVAD pump as well as other components that help power and monitor the technology including an external, wearable controller and battery system. The HeartMate 3 system received CE Mark in Europe for both short-term and long-term support in October 2015 and U.S. FDA approval for short-term support in August 2017.
For more information: www.heartmate.com/healthcare-provider/heartmate-3-lvad
Related Content on the HeartMate III:
Abbott Receives FDA Approval for HeartMate 3 Left Ventricular Assist System
VIDEO: Therapies for Advanced Heart Failure — interview with David Lanfear M.D., FACC, head of advanced heart failure and cardiac transplantation, Henry Ford Hospital, Detroit
Leading Cardiovascular Societies Release New Guidance on Use of Heart Pumps
References:


 May 30, 2025
May 30, 2025