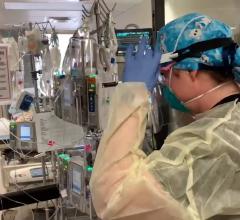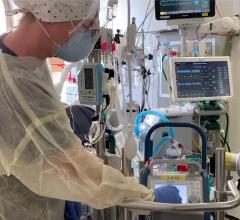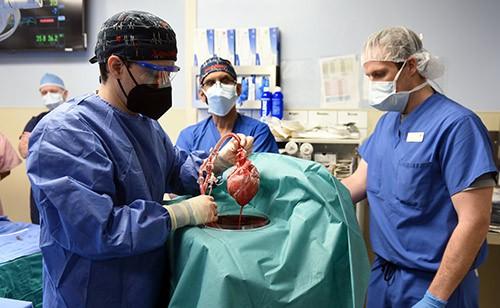
June 30, 2023 — A new study published in The Lancet has revealed the most extensive analysis to date on what led to the eventual heart failure in the world's first successful transplant of a genetically-modified pig heart into a human patient. This groundbreaking procedure was conducted by University of Maryland School of Medicine (UMSOM) physician-scientists back in January 2022 and marked an important milestone for medical science.
The patient, 57-year-old David Bennett, Sr., was treated at the University of Maryland Medical Center. He experienced strong cardiac function with no obvious signs of acute rejection for nearly seven weeks after the surgery. A sudden onset of heart failure led to his death two months after the transplant. Since then, the transplant team has been conducting extensive studies into the physiologic processes that led to the heart failure to identify factors that can be prevented in future transplants to improve the odds of longer-term success.
“Our paper provides crucial insight into how a multitude of factors likely played a role in the functional decline of the transplanted heart,” said study lead author Muhammad M. Mohiuddin, MD, Professor of Surgery and Scientific/Program Director of the Cardiac Xenotransplantation Program at UMSOM. “Our goal is to continue moving this field forward as we prepare for clinical trials of xenotransplants involving pig organs.”
Mr. Bennett, who was in end-stage heart failure and nearing the end of his life, did not qualify for a traditional heart transplant. The procedure was authorized by the U.S. Food and Drug Administration under its expanded access (compassionate use) provision.
“We were determined to shed light on what led to the heart transplant dysfunction in Mr. Bennett, who performed a heroic act by volunteering to be the first in the world,” said study co-author Bartley Griffith, MD, Professor of Surgery and The Thomas E. and Alice Marie Hales Distinguished Professor in Transplantation at UMSOM. “We want our next patient to not only survive longer with a xenotransplant but to return to normal life and thrive for months or even years.”
To better understand the processes that led to dysfunction of the pig heart transplant, the research team performed extensive testing on the limited available tissues in the patient. They carefully mapped out the sequence of events that led to the heart failure demonstrating that the heart functioned well on imaging tests like echocardiography until day 47 after surgery.
The new study confirms that no signs of acute rejection occurred during the first several weeks after the transplant. Likely, several overlapping factors led to heart failure in Mr. Bennett, including his poor state of health prior to the transplant that led him to become severely immunocompromised. This limited the use of an effective anti-rejection regimen used in preclinical studies for xenotransplantation. As a result, the researchers found, the patient was likely more vulnerable to rejection of the organ from antibodies made by the immune system. The researchers found indirect evidence of antibody-mediated rejection based on histology, immunohistochemical staining and single cell RNA analysis.
The use of an intravenous immunoglobulin, IVIG, a drug that contains antibodies, may also have contributed to damage to the heart muscle cells. It was given to the patient twice during the second month after the transplant to help prevent infection, likely also triggering an anti-pig immune response. The research team found evidence of immunoglobulin antibodies targeting the pig vascular endothelium layer of the heart.
Lastly, the new study investigated the presence of a latent virus, called porcine cytomegalovirus (PCMV), in the pig heart, which may have contributed to the dysfunction of the transplant. Activation of the virus may have occurred after the patient’s anti-viral treatment regimen was reduced to address other health issues. This may have initiated an inflammatory response causing cell damage. However, there is no evidence that the virus infected the patient or spread to organs beyond the heart. Improved PCMV testing protocols have been developed for sensitive detection and exclusion of latent viruses for future xenotransplants.
Other UMSOM faculty co-authors of this study include: Avneesh K Singh, PhD, Alison Grazioli, MD, Kapil Saharia, MD, Tianshu Zhang, MD, and Christine Lau, MD.
“Valuable lessons can be learned from this groundbreaking surgery and the courageous first patient, Mr. Bennett, that will better inform us for future xenotransplants,” said UMSOM Dean Mark T. Gladwin, MD, Vice President for Medical Affairs, University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor. “In the future, our team of surgeon-scientists will utilize newly designed immune cell assays to monitor the patient more precisely in the days, weeks, and months following the xenotransplant. This will provide stricter control of the earliest signs of rejection and the promise of a truly lifesaving innovation.”
For more information: https://www.medschool.umaryland.edu/
Related Pig Heart Transplant Content:
Lab-grown Pig Heart Tissue Could Help Replace Live Animals in Heart Disease Research
Pig Heart Transplant Patient Continues to Thrive
First Human Receives a Pig Heart Transplant
Transplanting Pig Hearts Into Humans One Step Closer
FDA OKs Use New Version of Carmat Artificial Heart in U.S. Early Feasibility Study
Carmat Artificial Heart Shows Promising Automatic Regulation of Blood Flow
Heart in a Box Technology Expands Heart Transplant Window
FDA Clears SynCardia 50cc Temporary Total Artificial Heart as Bridge to Transplant
Stephanie’s Heart: The Story of Baby Fae
Hearts from donors who used illicit drugs or overdosed safe for transplant, cuts wait time
National Trends in Heart Donor Utilization Rates: Are We Efficiently Transplanting More Hearts?
Heart transplants from donors with hepatitis C may be safe and could help decrease organ shortage


 September 13, 2023
September 13, 2023