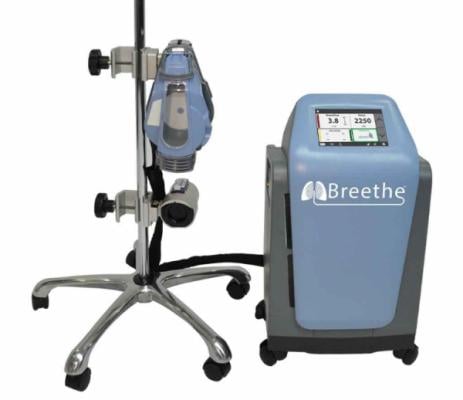
The first COVID-19 patient in the world was supported by the new Abiomed Breethe OXY-1 System at Hackensack University Medical Center.
February 25, 2021 — The first COVID-19 patient in the world was supported by the new Abiomed Breethe OXY-1 System, an advanced, compact, cardiopulmonary bypass system that pumps, oxygenates and removes carbon dioxide from the blood for patients whose lungs can no longer provide sufficient oxygenated blood.
The patient, a 51-year-old woman with respiratory failure due to COVID-19, began Breethe extracorporeal membrane oxygenation (ECMO) therapy Dec. 19, 2020. The patient was on a ventilator at the time of implant. The goal of Breethe therapy is to allow the lungs to heal and hopefully recover faster than ventilator therapy alone. After 24 hours of Breethe support, she was stable and improving.
She was treated by Yuriy Dudiy, M.D., attending cardiac surgeon and Mark Anderson, M.D., interim chair of the Department of Cardiac Surgery and a cardiothoracic surgeon at the Heart and Vascular Hospital at Hackensack Meridian Hackensack University Medical Center.
"Breethe is an important new option for patients with COVID-19 who require ECMO therapy. It is simple and intuitive to use, highly portable, and a very promising therapy with the potential to help many patients," Anderson said.
Patients are placed on the Breethe system temporarily to support their lung function by taking unoxygenated blood out of the body, circulating it through the oxygenator in the system, and pumping oxygenated blood back into the body to be distributed to the major organs. The system is only used in the hospital. Patients can be on support for days or weeks depending on their condition. Breethe supports lung recovery so patients can be weaned off the system and live with their native lungs without assistance.
The Breethe system received clearance from the U.S. Food and Drug Administration in October 2020. In addition to COVID-19, it can help provide oxygenation to patients suffering from cardiogenic shock or respiratory failure from acute respiratory distress syndrome (ARDS), H1N1 influenza or SARS. When used with the Impella heart pump, it can allow the heart to rest and oxygenate the body, a combination therapy known as ECpella.
"Hackensack Meridian Health has long been a leader in the implementation of the most advanced technologies based on the latest medical research, and our adoption of Breethe therapy is yet another example of our dedication to being at the forefront of patient care," added Ihor Sawczuk, M.D., FACS, Hackensack Meridian Health regional president, Northern Market and chief research officer.
"COVID-19 has had a significant impact on our communities, so everything we can do to fight back is a win. We welcome Breethe into our arsenal of therapies we use to support patients with COVID-19 who are fighting for their lives," said Mark Sparta, FACHE, president and chief hospital executive, Hackensack Meridian Hackensack University Medical Center and executive vice president of Population Health, Hackensack Meridian Health.
Related COVID-19 ECMO Support Content:
COVID Mother Reunited With Caregivers After Saving Her Live With ECMO
Abiomed Adds ECMO Cardiopulmonary Support to its Portfolio
FDA Allows Emergency Use of Impella for COVID-19 Patients on ECMO
VIDEO: ECMO Support Effective in Sickest COVID-19 Patients — Interview with Ryan Barbaro, M.D.,
FDA Approves ECMO to Treat COVID-19 Patients
VIDEO: COVID Survivor on ECMO in Hospital 152 Days Reunited With Clinicians Who Saved Her
ECMO Used to Treat Adult Respiratory Distress Syndrome Case
FDA Allows Emergency Use of Impella for COVID-19 Patients on ECMO

 March 20, 2024
March 20, 2024