The Breethe ECMO system. Abiomed invested in Breethe in mid-2019. Breethe has applied for 510(k) clearance by the Food and Drug Administration (FDA).
May 1, 2020 — Abiomed, maker of the Impella heart pump, has acquired Breethe, developer of a novel extracorporeal membrane oxygenation (ECMO) system that will complement and expand Abiomed’s product portfolio to more comprehensively serve the needs of patients whose lungs can no longer provide sufficient oxygenation. This includes patients suffering from cardiogenic shock or respiratory failure such as due to ARDS, H1N1, SARS, or COVID-19. ECMO has also been utilized as a primary method of oxygenation and hemodynamic support for pediatric patients.
Patients who require ECMO therapy have a severe and life-threatening illness that stops their lungs from working properly. The system is connected to patients through tubes (cannulae) and is an external respiratory assistance device that takes venous blood, removes carbon dioxide and adds oxygen, much like a human lung. Oxygenated blood is then sent back to the patient. Each year, more than 20,000 patients receive ECMO therapy in the United States.
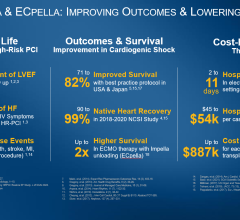
Abiomed recognizes the need for ECMO therapy for patients in need of oxygenation and has supported approximately 10,000 ECMO plus Impella (ECPella) patients with cardiogenic shock over the past 10 years. In Japan, more than half of Impella patients receive ECPella for hemodynamic and oxygenation support.
Abiomed recognizes the need for ECMO therapy for patients in need of oxygenation and has supported approximately 10,000 ECMO plus Impella (ECPella) patients with cardiogenic shock over the past 10 years.
Breethe’s product is a first-of-its kind, easy-to-use compact ECMO system with an integrated oxygen concentrator that eliminates the need for bulky oxygen tanks to promote easier patient ambulation. It has a novel design that is intuitive for health care providers to set up, manage, and monitor. Abiomed invested in Breethe in mid-2019. Breethe has applied for 510(k) clearance by the Food and Drug Administration (FDA).
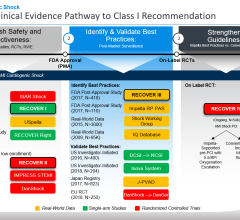
Breethe’s founder and the Hales Distinguished Professor of Surgery at University of Maryland School of Medicine, Bartley Griffith, M.D., is a renowned leader in multiple areas of adult cardiac surgery including mechanical circulatory support. Dr. Griffith has collaborated with Abiomed for a number of years and served as the principal investigator of RECOVER I trial, which looked at the Impella 5.0 system. With decades of experience, Dr. Griffith and his team designed the cutting-edge Breethe system to improve patient outcomes, improve quality of life and reduce total cost of care by changing the way oxygenation is delivered.
“Abiomed is the best positioned company to build on the legacy of what we started,” Griffith said. “I am confident that the addition of Breethe’s technology into Abiomed’s product portfolio will further enhance Abiomed’s ability to improve outcomes for their patients and serve a new patient population.”
“This acquisition is a natural progression toward improving patient care,” said Matthew D. Bacchetta, M.D., associate professor, Department of Thoracic Surgery at Vanderbilt University. “Breethe’s compact and all-in-one technology aims for improved patient ambulation, which can improve outcomes and promote active rehabilitation for patients with cardiopulmonary diseases.”
“Breethe will integrate with Abiomed and our manufacturing, quality, sales, engineering and research capabilities, including Abiomed’s best-in-class Clinical Support Center,” said Michael R. Minogue, Abiomed’s chairman, president and CEO. “This acquisition aligns with our principles of leading in technology and innovation, putting patients first and striving to continually improve outcomes. Physicians have asked Abiomed to bring this technology into our portfolio because of our ability to support patients, teach best practices, and collect critical data for research. This ECMO technology will allow us to treat cardiogenic shock patients who are already being supported with Impella, add pediatric offerings and treat a new patient population with respiratory failure.”
This acquisition provides Abiomed with the opportunity to innovate traditional ECMO technology, focusing on patient ambulation and recovery from acute respiratory failure. For many patients in cardiogenic shock, Impella is the optimal technology because it unloads the left ventricle, perfuses end organs and allows the heart to rest and recover. Abiomed recognizes patients in cardiogenic shock may also need oxygenation. ECMO perfuses the end organs but does not unload the left ventricle, which increases the oxygen demand of the myocardium in these patients. For patients in cardiogenic shock, Impella with ECMO work together to unload the heart and oxygenate the body.
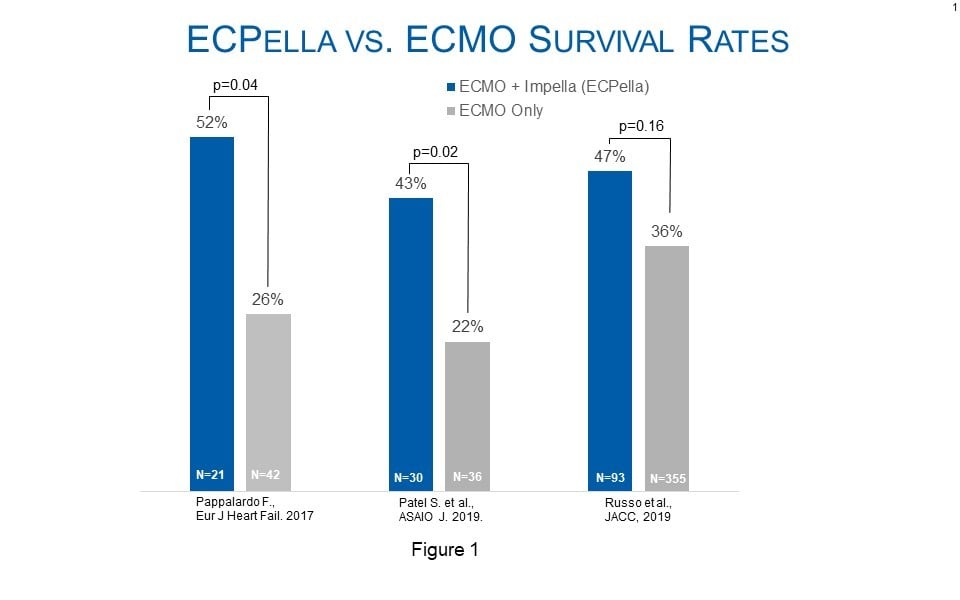
Multiple studies support the association of ECPella therapy to improve outcomes for patients who are suffering from cardiogenic shock and require oxygenation. The European Journal of Heart Failure, ASAIO, and the Journal of the American College of Cardiology have published studies that examine a combined 4,126 patients and conclude use of ECPella was associated with increased survival rates, as compared to patients who were treated with ECMO only. In addition to higher survival, the study in the European Journal of Heart Failure by Pappalardo et al. found higher rates of heart recovery with ECPella use than with ECMO only (62 vs 36 percent; p=0.048).
“As the number of COVID-19 cases surge, so does the demand for extracorporeal membrane oxygenation (ECMO) therapy. Through the acquisition of Breethe and its ECMO system, Abiomed is hoping to help fill this demand. Breethe has applied for FDA 510(k) clearance for the system," said Azadeh Laffafian Ph.D., a medical device analyst at GlobalData, a healthcare market data and analytics company. “The ECMO system will be a natural addition to Abiomed’s product portfolio going forward. ECMO therapy can be used in combination with Abiomed’s Impella heart pumps to treat patients with cardiogenic shock."
According to Abiomed’s quarterly results, Laffafian said the COVID-19 pandemic has had a negative effect on sales of Impella heart pumps. The company estimates that it lost $17 million in revenue due to the pandemic.
Terms of the acquisition are not being disclosed at this time.


 April 28, 2023
April 28, 2023