October 21, 2022 — Abiomed announced the U.S. Food and Drug Administration (FDA) has accepted and closed the post-approval study reports related to the pre-market approvals (PMA) for Impella heart pumps. The FDA’s action is another affirmation that Impella heart pumps are safe and effective for cardiogenic shock, high-risk PCI, post-cardiotomy cardiogenic shock, cardiogenic shock in the setting of myocarditis or cardiomyopathy, and right heart failure.
The FDA typically requires post-approval studies for medical devices that receive a PMA, the FDA’s highest level of regulatory approval. FDA post-approval studies use high-quality prospective data to confirm that the clinical study data submitted to the FDA to receive a PMA applies to a broader, real-world population of patients.
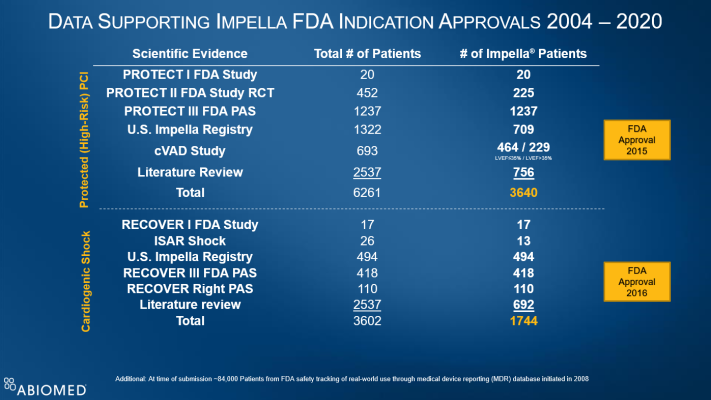
In total, Abiomed completed five post-approval studies for Impella over the seven years since its initial PMA was received. This large, multi-center experience was conducted at 46 sites and enrolled a total of 1,833 patients.
“This significant regulatory milestone once again confirms the safety and efficacy of Impella across a variety of clinical indications. I applaud the physician-researchers who led these studies and thank the patients who participated in them,” said Chuck Simonton, MD, Abiomed’s chief medical officer.
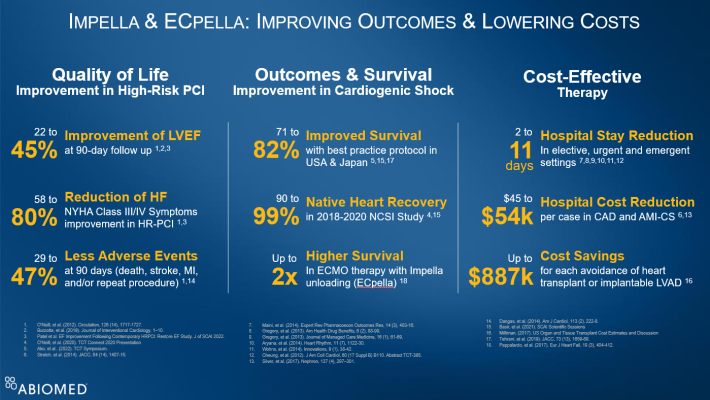
The totality of Impella data collected in the United States, Europe and Japan demonstrates Impella improves outcomes and lowers costs. (see figure 1)
This Data Includes:
- Impella-supported Protected PCI improves quality of life, with a 22% to 45% improvement in left ventricular ejection fraction at 90-day follow up1, 2, 3, a 58% to 80% reduction in New York Heart Association Class III and IV symptoms1, 3 and 29% to 47% fewer adverse events at 90 days1, 14.
- Impella improves outcomes in cardiogenic shock, with 71% to 82% survival with best practice protocols5, 15, 17, 90% to 99% native heart recovery in the 2018 to 2020 National Cardiogenic Shock Initiative Study4, 15 and up to two-times higher survival for ECMO therapy when it is combined with Impella unloading (known as ECpella)18.
- Impella is a cost-effective therapy that reduces hospital length-of-stay two to eleven days in elective, urgent and emergent settings7, 8, 9, 10, 11, 12, reduces hospital cost per case $45,000 to $54,000 in coronary artery disease and AMI cardiogenic shock6, 13 and provides up to $887,000 in cost savings for each avoidance of a heart transplant or implantable LVAD16.
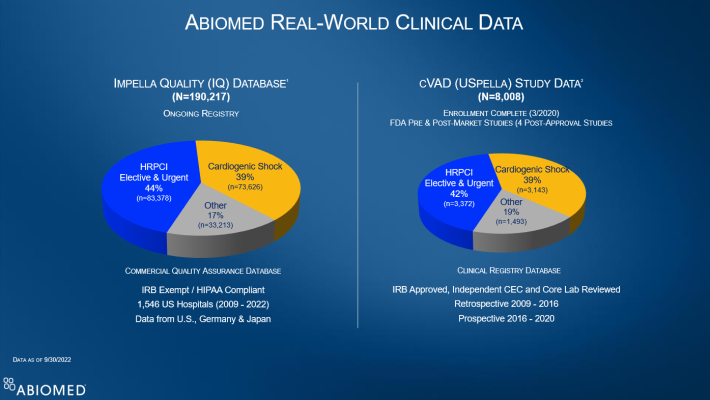
Impella is the most studied heart pump in the history of the FDA, with studies being conducted from 2006 to the present. Real world data exists on nearly 200,000 Impella patients (see figures 2 & 3) and Impella is the subject of more than 1,200 peer-reviewed publications. It is included in 13 clinical society guidelines.
The clinical data and best practices learned from all Impella studies performed with the FDA, the Japanese Pharmaceuticals and Medical Devices Agency and in Europe, combined with prospective and real world data, informed the design of the PROTECT IV and RECOVER IV randomized controlled trials. These on-label trials are designed to achieve the level of evidence for Impella to receive Class I guideline recommendations for high-risk PCI and AMI cardiogenic shock.
For more information: www.abiomed.com
References:
- O’Neill, et al. (2012). Circulation, 126 (14), 1717-1727.
- Burzotta, et al. (2019). Journal of Interventional Cardiology, 1–10.
- Patel et al. EF Improvement Following Contemporary HRPCI: Restore EF Study. J of SCAI 2022.
- O’Neill, et al. (2020). TCT Connect 2020 Presentation.
- Ako, et al.. (2022). TCT Symposium.
- Stretch, et al. (2014). JACC, 64 (14), 1407-15.
- Maini, et al. (2014). Expert Rev Pharmacoecon Outcomes Res, 14 (3), 403-16.
- Gregory, et al. (2013). Am Health Drug Benefits, 6 (2), 88-99.
- Gregory, et al. (2013). Journal of Managed Care Medicine, 16 (1), 61-69.
- Aryana, et al. (2014). Heart Rhythm, 11 (7), 1122-30.
- Wohns, et al. (2014). Innovations, 9 (1), 38-42.
- Cheung, et al. (2012). J Am Coll Cardiol, 60 (17 Suppl B) B110. Abstract TCT-385.
- Silver, et al. (2017). Nephron, 137 (4), 297–301.
- Dangas, et al. (2014). Am J Cardiol, 113 (2), 222-8.
- Basir, et al. (2021). SCAI Scientific Sessions.
- Milliman. (2017). US Organ and Tissue Transplant Cost Estimates and Discussion.
- Tehrani, et al. (2019). JACC, 73 (13), 1659-69.
- Pappalardo, et al. (2017). Eur J Heart Fail, 19 (3), 404-412.
Related content:
Consent FDA Approves RECOVER IV Randomized Controlled Trial with Exception from Informed (EFIC)
For more information on current best practices in treating AMI cardiogenic shock patients: click here
For more information on the RECOVER IV RCT study: click here


 February 03, 2026
February 03, 2026 









