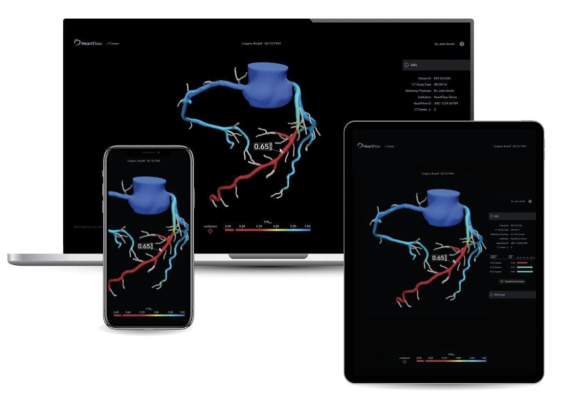
October 18, 2022 — HeartFlow, Inc., a leader in revolutionizing precision heart care, has received U.S. Food and Drug Administration (FDA) 510(k) clearance on two new, AI-powered products: Plaque analysis and RoadMap analysis. With its expanded product portfolio, HeartFlow is the first and only company to provide non-invasive coronary artery anatomy (RoadMap analysis), physiology (HeartFlow FFRCT), and plaque information (Plaque analysis) based on CCTA. These products enable physicians to gain a more comprehensive understanding of a patient’s coronary artery disease (CAD) and are the most accurate approach to predict risk of a heart attack.1
“The 510(k) clearance of our Plaque and RoadMap analyses represents a major milestone in the company’s commitment to provide physicians with richer clinical insights to help diagnose and treat individual patients, no matter where they are on the coronary disease spectrum,” said John Farquhar, President and Chief Executive Officer, HeartFlow. “FFRCT has already been recognized by the recent ACC/AHA Chest Pain Guidelines and is poised to change the standard-of-care in patients. Plaque and RoadMap analyses, together with FFRCT, establish HeartFlow’s platform technologies and will enable further development of AI-powered risk scoring to better identify asymptomatic patients at risk of heart attack.”
HeartFlow’s Plaque analysis has been studied in 11,000+ patients and will provide physicians with comprehensive and actionable data showing plaque characteristics and volume in all major coronary arteries.2 It enables critical information regarding coronary plaque to be delivered conveniently to physicians along with anatomy and physiology.3 Centers for Medicare & Medicaid Services recently announced that, as of October 1, 2022, the plaque analysis is paid as a separate service in the hospital outpatient department.
The RoadMap analysis will enable CT readers to improve CAD diagnosis by providing visualization and quantification of the location and severity of anatomic narrowings in the coronary arteries on every CCTA. It has been shown to provide reproducible results and can help enable efficient, standardized, and high quality CT interpretation.4
“It is exciting to note the work HeartFlow is doing to bring forward the innovative technologies to help us advance our understanding and care for patients with coronary artery disease. Combining anatomy, physiology, and plaque morphology would be essential for personalized patient care,” said Jagat Narula, MD, PhD, Chief of Cardiology at Mount Sinai Morningside Hospital.
With this FDA 510(k) clearance, HeartFlow will begin real-world clinical use of the Plaque and RoadMap analyses with select hospitals and health systems. HeartFlow’s latest technologies will play a vital role in improving cardiovascular patient care.
For more information: www.heartflow.com
Related Chest Pain Imaging Content:
First International Chest Pain Diagnosis Guidelines Released
VIDEO: Why the ASNC Did Not Endorse the 2021 Chest Pain Guidelines — Interview with ASNC President Dennis Calnon, M.D.


 November 14, 2025
November 14, 2025 









