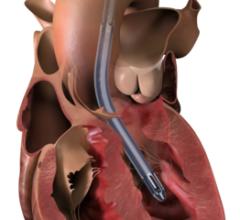
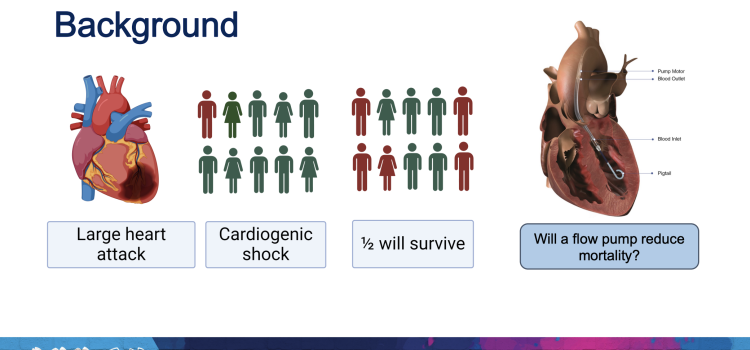
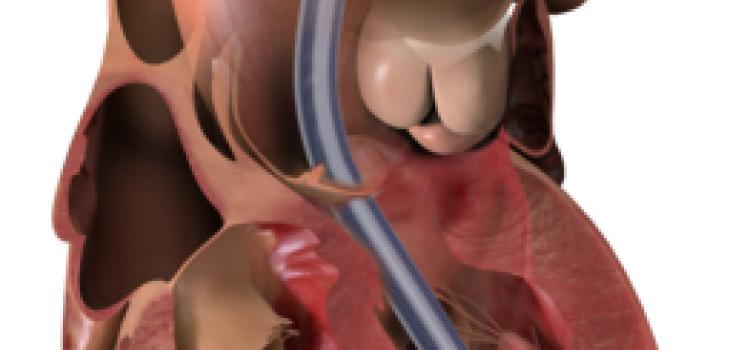
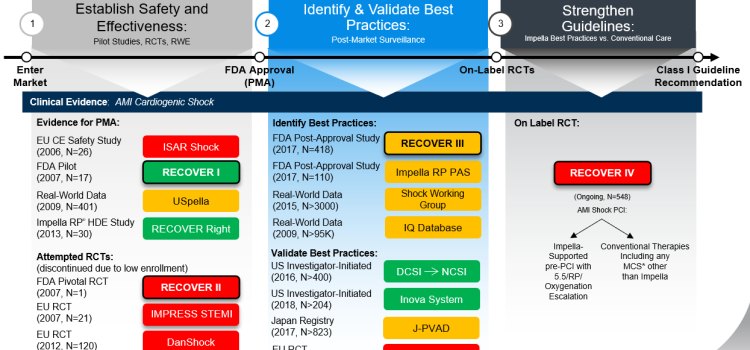
April 8, 2024 — Implantation of the Impella CP micro-axial flow pump in the hours after a heart attack significantly increased the rate of survival at six months among people suffering cardiogenic ...
Cardiogenic Shock
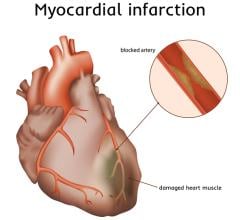
This page includes content on technologies to treat cardiogenic shock, including percutaneous ventricular assist devices (pVAD), intra-aortic balloon pumps (IABP) and extracorporeal membrane oxygenation (ECMO). This condition occurs when the heart can no longer pump enough oxygenated blood to the body. The most common cause of cardiogenic shock is damage to the heart from a severe heart attack. The standard of care for this condition for more than 20 years has a 50 percent survival rate, but improvements have recently been seen in studies using early pVAD intervention prior to percutaneous coronary intervention (PCI). For more information, visit the National Institute of Health (NIH) cardiogenic shock information page.
April 8, 2024 — Implantation of the Impella CP micro-axial flow pump in the hours after a heart attack significantly ...
October 26, 2023 — A new study presented at TCT 2023 revealed inequities in access to mechanical circulatory support in ...
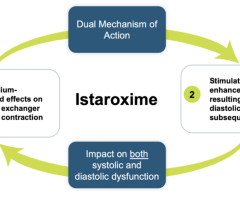
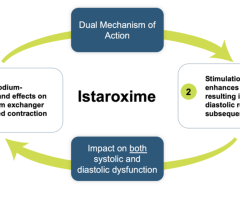
April 28, 2023 — Windtree Therapeutics, Inc. is a biotechnology company focused on advancing late-stage interventions ...
March 22, 2023 — Windtree Therapeutics, Inc., a biotechnology company focused on advancing multiple late-stage ...
March 15, 2023 — Windtree Therapeutics, Inc, a biotechnology company focused on advancing late-stage interventions for ...
November 7, 2022 — The immediate use of veno-arterial mechanical circulatory extracorporeal membrane oxygenation (ECMO) ...
October 20, 2022 — Cardiogenic shock—a life threatening condition when a person’s heart can’t pump enough blood to meet ...
September 27, 2022 — Windtree Therapeutics, Inc., a biotechnology company focused on advancing multiple late-stage ...
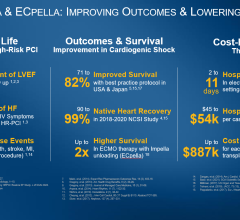
September 22, 2022 — Abiomed (ABMD) announces the result of a three-year, investigator-led study of all Impella ...
September 20, 2022 — An analysis from the Society of Thoracic Surgeons/American College of Cardiology(STS/ACC) TVT ...
September 19, 2022 — Supira Medical, Inc., a Shifamed portfolio company focused on developing the next-generation ...
September 16, 2022 — Abiomed announced two approvals from the U.S. Food and Drug Administration (FDA) related to ...
September 2, 2022 — Acetazolamide added to intravenous loop diuretics decreases congestion within three days in patients ...
June 1, 2022 — Recardio's Phase 2 trial results demonstrated the excellent safety profile of its lead drug Dutogliptin ...


 April 08, 2024
April 08, 2024