April 28, 2023 — Windtree Therapeutics, Inc. is a biotechnology company focused on advancing late-stage interventions for acute cardiovascular disorders of acute heart failure and a lead program studying istaroxime in cardiogenic shock. Cardiogenic shock is caused by a failing heart resulting in diminished cardiac output to the body and is characterized by very low blood pressure and hypoperfusion to end-organs. It is a treatment emergency with high morbidity and mortality.
Windtree conducted a study of istaroxime in patients experiencing early cardiogenic shock due to heart failure (the SEISMiC Study) and previously reported the publication of the primary analysis of SEISMiC comparing the combined istaroxime dose groups and placebo. In this study the primary endpoint of systolic blood pressure area under the curve (SBP AUC) over 6 hours was significantly improved by istaroxime compared to the placebo control group, an effect that was maintained throughout the 24 hour infusion. Patients treated with istaroxime experienced a substantial increase in stroke volume (the amount of blood pumped from the heart with each contraction) that contributed to increased cardiac output without increasing heart rate. As also reported, the study met several other secondary endpoint assessments of cardiac function and importantly, demonstrated that renal function did not worsen in the istaroxime treated group.
Today, Windtree announces the publication of “Safety and Efficacy of Istaroxime 1.0 and 1.5 μg/kg/min for Patients with Pre Cardiogenic Shock,” in the Journal of Cardiac Failure. This publication describes additional analysis completed on dosing of istaroxime in the SEISMiC Study and conveys dose related findings on the primary endpoint SBP AUC and on biomarkers and clinical endpoints. This publication describes both doses of istaroxime as increasing SBP, with the 1.0 μg/kg/min dose having a numerically greater SBP AUC change (93.6% vs 39.5% relative increase over the first 6 hours of the infusion) and fewer serious adverse events than the 1.5 μg/kg/min dose. It also notes that echocardiographic assessment of cardiac function was similar between doses with both doses showing improvement.
“This analysis of our SEISMiC Phase 2 study in pre-cardiogenic shock has provided valuable dose related information,” said Dr. Steve Simonson, SVP and Chief Medical Officer of Windtree Therapeutics. “This publication describes the benefit derived from the 1.0 μg/kg/min dose and indicates the desired improvements in cardiac function and blood pressure may be achieved without requiring the administration of higher doses of istaroxime. These findings will guide our future development strategy in cardiogenic shock, including in the extension study to SEISMiC, which focuses on dose optimization and longer infusions.”
About Istaroxime
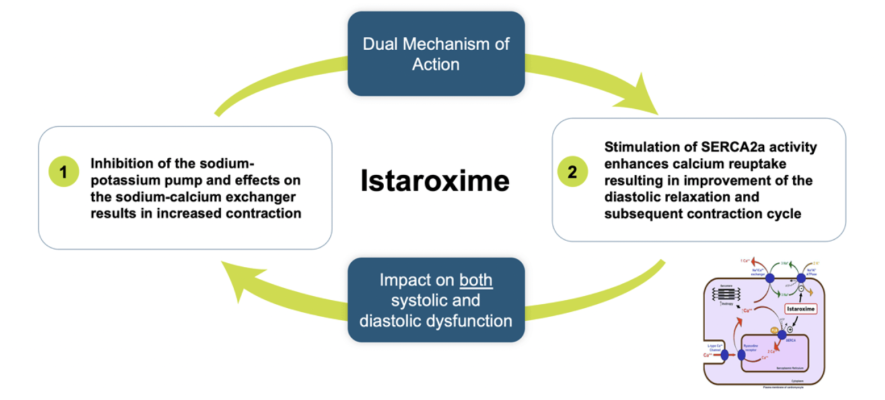
Istaroxime is a first-in-class dual mechanism therapy designed to improve both systolic and diastolic cardiac function. Istaroxime is a positive inotropic agent that increases myocardial contractility through inhibition of Na+/K+- ATPase with a complimentary mechanism that facilitates myocardial relaxation through activation of the SERCA2a calcium pump on the sarcoplasmic reticulum enhancing calcium reuptake from the cytoplasm. Data from multiple Phase 2 studies in patients with acute heart failure (AHF) demonstrate that istaroxime infused intravenously significantly improves cardiac function and blood pressure without increasing heart rate or the incidence of cardiac rhythm disturbances.
For more information: https://windtreetx.com/
Related Cardiogenic Shock Content:
Windtree Therapeutics Announces Issuance of New Dual Mechanism SERCA2a Activator Patent
Windtree Completes Enrollment of Phase 2 Study of Istaroxime in Early Cardiogenic Shock


 January 05, 2026
January 05, 2026 









