Image credit: Getty Images
March 31, 2022 – Windtree Therapeutics, a biotechnology company focused on advancing multiple late-stage interventions for acute cardiovascular and pulmonary disorders, today announced it has successfully completed enrollment in its phase 2 study of istaroxime in early cardiogenic shock caused by heart failure.
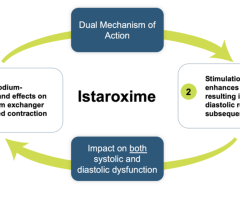
The study is an international, randomized double-blind, placebo-controlled study designed to assess the efficacy and safety of istaroxime and to support an intended pathway for the development in early cardiogenic shock. The study has enrolled 60 Society for Cardiovascular Angiography & Interventions (SCAI) class B early cardiogenic shock patients with severe heart failure (30 assigned to istaroxime and 30 assigned to placebo) and systolic blood pressures (SBP) between 75-90 mmHg. Study drug was administered over 24 hours. The primary endpoint is the SBP profile over the first 6 hours after initiating the infusion. Secondary endpoints will include various assessments of blood pressure changes over 24 hours and measures associated with safety and tolerability. All patients will complete a 30-day follow-up prior to database lock and generation of topline data; as a result, topline data is expected to be announced in April.
“We are very pleased to have completed enrollment in the study of istaroxime in early cardiogenic shock due to heart failure. We are eager to examine the potential of istaroxime in this critical condition. The efforts of participating centers to enroll patients are much appreciated given the challenges of conducting research in hospital cardiac care units during the global pandemic,” said Steve Simonson, MD, CMO of Windtree Therapeutics.
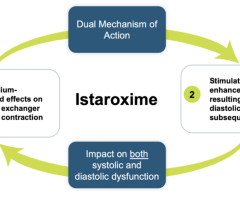
Cardiogenic shock is a serious condition that occurs when the heart is failing significantly and cannot pump enough blood and oxygen to the brain, kidneys, and other vital organs. Mortality rates are significant and, depending on severity, range from 7% to 40% in the U.S. There is a lack of satisfactory pharmacological intervention to reverse the condition as available therapies have unwanted side effects such as risk for arrhythmias, decreasing blood pressure, renal dysfunction and even increases in mortality that limit their usefulness and position them as “rescue medicines” for severe cases. Market research revealed 99% of 100 U.S.-based clinical cardiologists interviewed who treat cardiogenic shock patients responded that new drug innovation to treat SCAI class B cardiogenic shock patients is highly needed. The cardiogenic shock worldwide total market value is estimated to be $1.25 billion.
Craig Fraser, CEO and President of Windtree Therapeutics added, “Early cardiogenic shock patients need new therapies that can be used earlier and more broadly to rapidly improve blood pressure and cardiac function without many of the unwanted side effects of existing, older agents that often cause cardiologists to reserve using them. We look forward to announcing topline data and our continued execution of this program, as well as the larger acute heart failure program, as we move forward in developing istaroxime as an innovative new therapy.”
For more information: https://windtreetx.com/

 April 28, 2023
April 28, 2023