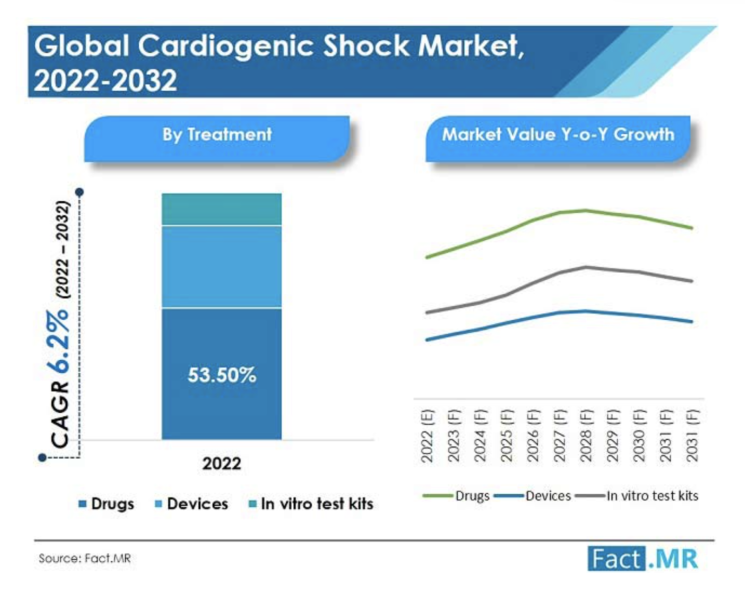
The global cardiogenic shock market was valued at US$ 3.29 Bn in 2021, and is expected to reach US$ 6.32 Bn by 2032 ...
Cardiogenic Shock
This page includes content on technologies to treat cardiogenic shock, including percutaneous ventricular assist devices (pVAD), intra-aortic balloon pumps (IABP) and extracorporeal membrane oxygenation (ECMO). This condition occurs when the heart can no longer pump enough oxygenated blood to the body. The most common cause of cardiogenic shock is damage to the heart from a severe heart attack. The standard of care for this condition for more than 20 years has a 50 percent survival rate, but improvements have recently been seen in studies using early pVAD intervention prior to percutaneous coronary intervention (PCI). For more information, visit the National Institute of Health (NIH) cardiogenic shock information page.
March 31, 2022 – Windtree Therapeutics, a biotechnology company focused on advancing multiple late-stage interventions ...
March 22, 2022 – Women are less likely to receive lifesaving treatment for cardiogenic shock than men, according to ...
Srihari Naidu, M.D., FACC, FAHA, FSCAI, is the director of both the cardiac cath labs and Hypertrophic Cardiomyopathy ...
April 29, 2021 — The results of a large, national heart attack study show that patients with cardiogenic shock survived ...
William O’Neill, M.D., medical director of the Center for Structural Heart Disease at Henry Ford Hospital, Detroit ...
October 26, 2020 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to Abiomed for an all-in-one ...
Chuck Simonton, M.D., chief medical officer at Abiomed, discusses some of the new technologies and clinical trials the ...
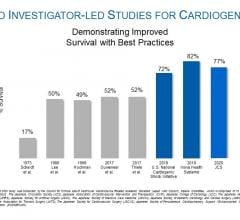
August 3, 2020 — A three-year, investigator-led, prospective study of Japanese patients who received an Impella heart ...
Navin Kapur, M.D., FAHA, FACC, FSCAI, director, Acute Mechanical Circulatory Support Program and executive director of ...
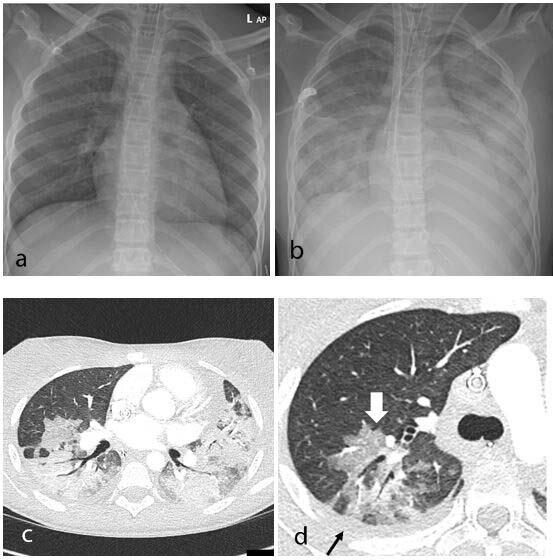
In recent weeks, a multisystem hyperinflammatory condition has emerged in children in association with prior exposure or ...
It was originally thought novel coronavirus (COVID-19, SARS-CoV-2) was primarily a respiratory disorder, but as larger ...
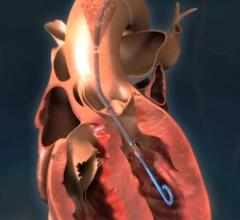
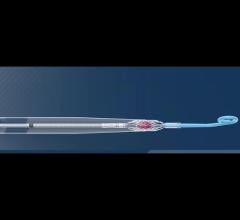
This is a quick animation demonstrating how the new 9 French Abiomed Impella ECP expands to approximately 18 French and ...
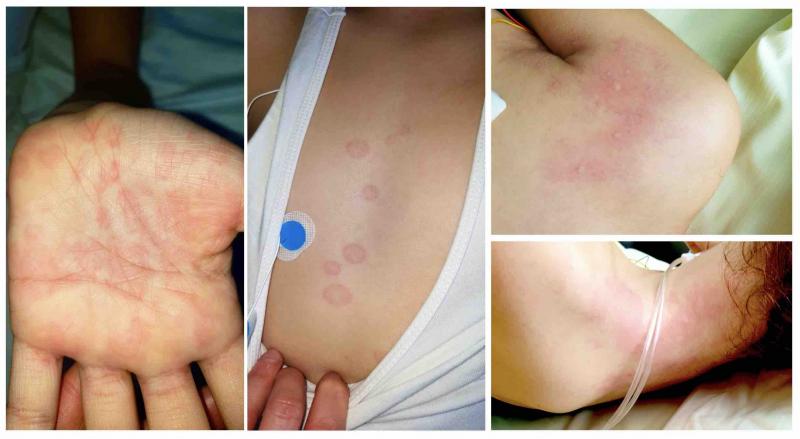
A new, serious COVID-19 cardiovascular presentation emerged in late April and early May 2020 in the form of pediatric ...
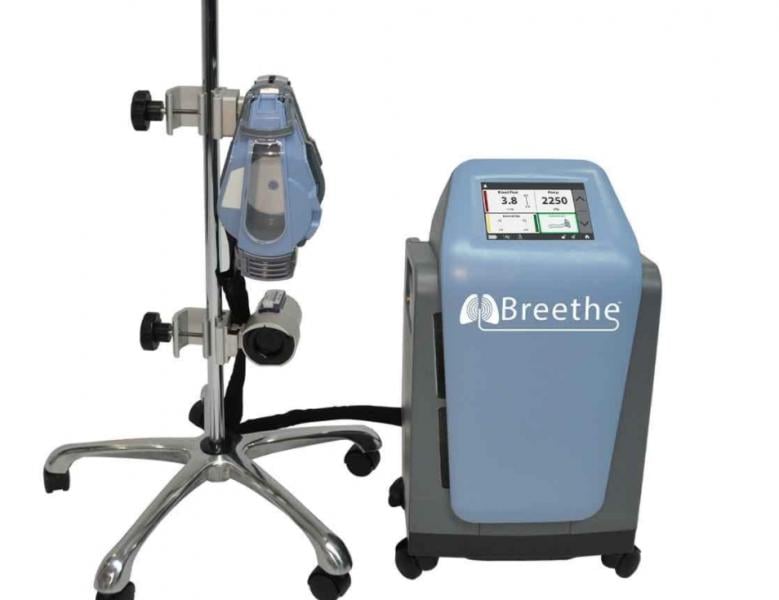
May 1, 2020 — Abiomed, maker of the Impella heart pump, has acquired Breethe, developer of a novel extracorporeal ...

 May 12, 2022
May 12, 2022