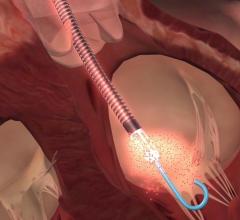
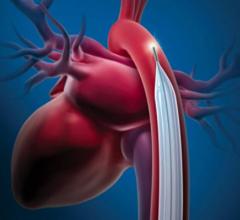
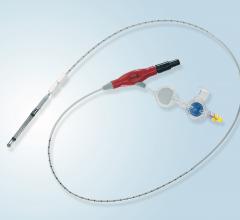
April 23, 2020 – LivaNova PLC said the U.S. Food and Drug Administration (FDA) is now permitting the company to expand ...
Cardiogenic Shock
This page includes content on technologies to treat cardiogenic shock, including percutaneous ventricular assist devices (pVAD), intra-aortic balloon pumps (IABP) and extracorporeal membrane oxygenation (ECMO). This condition occurs when the heart can no longer pump enough oxygenated blood to the body. The most common cause of cardiogenic shock is damage to the heart from a severe heart attack. The standard of care for this condition for more than 20 years has a 50 percent survival rate, but improvements have recently been seen in studies using early pVAD intervention prior to percutaneous coronary intervention (PCI). For more information, visit the National Institute of Health (NIH) cardiogenic shock information page.

DAIC Editor Dave Fornell recently took a tour of the Abiomed Impella production line at its headquarters in Danvers ...
Navin Kapur, M.D., FAHA, FACC, FSCAI, director, Acute Mechanical Circulatory Support Program and executive director of ...
Haval Chweich, M.D., medical director of the cardiac critical care unit (CCU) at Tufts Medical Center, and assistant ...
November 19, 2019 — Since Maquet/Datascope first recalled all of its intra-aortic balloon pumps (IABP) due to reports ...
November 4, 2019 — Abbott is recalling its CentriMag Acute Circulatory Support System due to a calibration system error ...
William O’Neill, M.D., medical director of the Center for Structural Heart Disease at Henry Ford Hospital, Detroit ...

Henry Ford Hospital thought leaders regularly speak at the cardiology conferences about new research and technology ...
There was a 77 percent increase in survival in cardiogenic shock patients treated using a new protocol in the National ...
A discussion with William O'Neill, M.D., director of the structural heart program, Henry Ford Hospital, and Michele ...
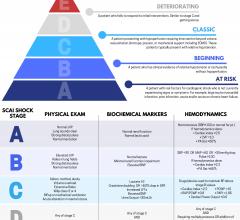
May 20, 2019 – A newly released expert consensus statement proposes a classification schema for cardiogenic shock (CS) t ...
May 14, 2019 — The U.S. Food and Drug Administration (FDA) has approved the expansion of Abiomed’s Impella 5.0 and ...
This podcast is a discussion with William O'Neill, M.D., director of the structural heart program, Henry Ford Hospital ...
Clinical study data makes the world go around in cardiology and is the basis of setting guidelines in evidence-based ...
William O'Neill, M.D., highlights best practice protocols based on Impella Quality database and real-world evidence ...


 April 23, 2020
April 23, 2020