May 14, 2019 — The U.S. Food and Drug Administration (FDA) has approved the expansion of Abiomed’s Impella 5.0 and Impella LD premarket approval (PMA) labeling for the treatment of cardiogenic shock. The expansion extends the duration of support for each pump from 6 days to 14 days.
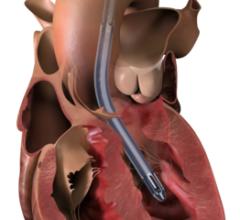
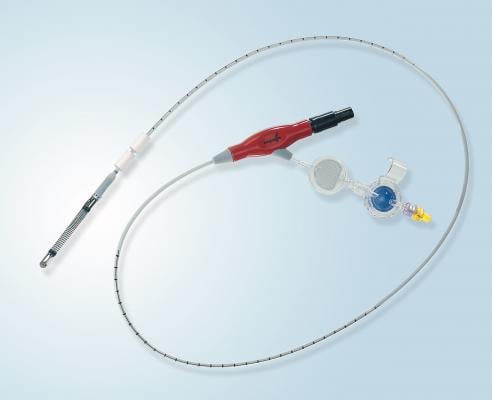
The Impella 5.0 and the Impella LD are forward flow heart pumps that deliver up to 5 L/min, stabilizing a patient’s hemodynamics, unloading the left ventricle and perfusing the end organs, allowing for the potential of native heart recovery or return to heart function baseline. The Impella 5.0 is implanted through the femoral or axillary artery, and the Impella LD is implanted directly into the aorta. Both allow patients to walk around the unit while on support.
Impella heart pumps have FDA PMA approval to treat heart attack patients in cardiogenic shock, and for shock associated with peripartum cardiomyopathy or myocarditis. They have the ability to enable the heart to rest and recover, allowing patients to return home with their own heart. The expanded indication allows for the opportunity to provide longer duration of support for critically ill patients and a longer period of assessment of heart recovery.
One patient who benefited from Impella 5.0 support is Erin Hanussak, then 33 years old, who suffered from myocarditis and went into cardiogenic shock. Her ejection fraction was 15 percent. Under the care of Jacob Abraham, M.D., a heart failure cardiologist at Providence St. Vincent Medical Center and medical director of its Center for Advanced Heart Disease, physicians implanted the Impella CP heart pump. Although Hanussak’s condition improved, the team determined that her heart needed additional support. Surgeons inserted the Impella 5.0 into her axillary artery, which allowed Erin to begin physical therapy and walk the hospital corridors with hospital staff. Her kidneys began to improve and after 12 days, the Impella 5.0 was explanted. Erin returned home to her family with her native heart and is back to her busy life as a mom.
“Early recognition, escalation and a heart team approach are crucial for patients in cardiogenic shock,” said Abraham. “The Impella 5.0 and Impella LD’s ability to provide greater hemodynamic support and unload the left ventricle make them ideal tools for patients like Erin who need longer duration support and will benefit from ambulation.”
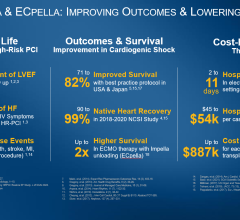
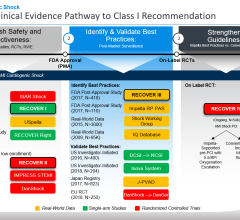
The FDA’s original PMA approval indicating Impella as safe and effective for the treatment of cardiogenic shock was granted in 2016. This approval was based on an analysis of 415 patients from the FDA study RECOVER 1 and the U.S. Impella registry, and an Impella literature review of 692 patients in 17 clinical studies. Additionally, more than 24,000 Impella patients supported by Impella devices were reviewed in a safety analysis.
For more information: www.abiomed.com

 April 28, 2023
April 28, 2023