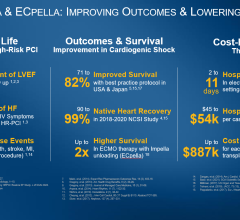
The Abiomed Impella percutaneous heart pump was shown in the NCSI study to greatly improve survival of acute myocardial infarction with cardiogenic shock (AMICS), raising survival from 50 percent to 71 percent. Ambulance image from Getty Images.
April 29, 2021 — The results of a large, national heart attack study show that patients with cardiogenic shock survived at a significantly higher rate when treated with a protocol using the transcatheter Impella heart pump to begin early and significant hemodynamic support. Survival increased from around 50% to 71% using the protocol.
“Cardiogenic shock is the leading cause of death in heart attack patients and outcomes have not improved over the past two decades,” said lead author Babar Basir, D.O., director of acute mechanical circulatory support at Henry Ford Health System in Detroit. “With early use of mechanical cardiac support, coupled with hemodynamic monitoring, we have the potential to increase survival to 80%, and save 20,000 life per year in the United States.”
The final results from the National Cariogenic Shock Initiative (NCSI) study were presented as a late-breaking trial at the Cardiovascular Angiography and Interventions (SCAI) 2021 Scientific Sessions this week.
The protocol was developed by cardiologists at Henry Ford Hospital in collaboration with four metro Detroit hospitals in a local effort called the Detroit Cariogenic Shock Initiative. Their success spurred the expanded National Cariogenic Shock Initiative (NCSI) in 2017 with sites across the country.
Cardiogenic shock is a complication that affects about 5-8 percent of heart attack victims in the United States annually. In these patients, the pump function of the heart is severely depressed, causing low blood pressure that leads to organ shut down due to the lack of perfusion. The new NCSI approach is to use of rapid, high-volume mechanical circulatory support (MCS) to maintain organ perfusion before and during percutaneous coronary intervention (PCI) in acute myocardial infarction and cardiogenic shock (AMICS) patients.
“The National Cardiogenic Shock Initiative is the largest prospective study of therapy for acute myocardial infarction cardiogenic shock done in the United States in the past 20 years,” said William O’Neill, M.D., medical director of Henry Ford’s Center for Structural Heart Disease and principal investigator of the study.
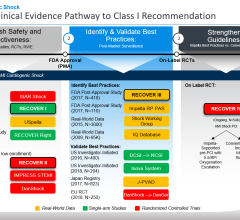
 “We have found that the original observations of the Detroit Cardiogenic Shock Initiative have been reproduced in 80 hospitals throughout the U.S.," O'Neill explained. "If implemented across the country, the National Cardiogenic Shock Initiative protocol could save up to 20,000 lives a year. We strongly believe that our results will be validated in the upcoming Recover IV trial, which should commence enrollment late next year.”
“We have found that the original observations of the Detroit Cardiogenic Shock Initiative have been reproduced in 80 hospitals throughout the U.S.," O'Neill explained. "If implemented across the country, the National Cardiogenic Shock Initiative protocol could save up to 20,000 lives a year. We strongly believe that our results will be validated in the upcoming Recover IV trial, which should commence enrollment late next year.”
Hear more about the NCSI study results in the VIDEO: Final Results of the National Cardiogenic Shock Initiative Show 72 Percent Survival — Interview with William O’Neill, M.D. at SCAI 2021
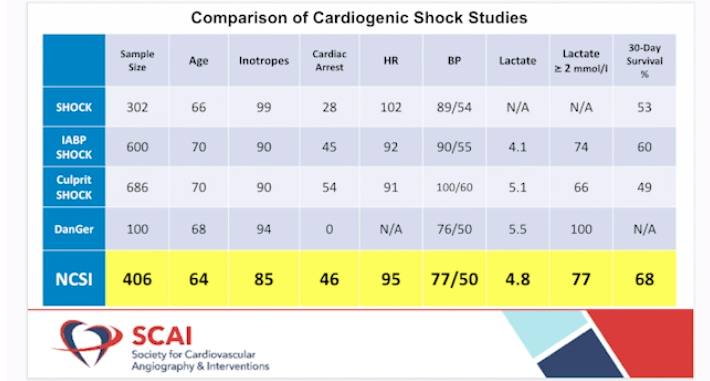
Eighty hospitals in 29 states participated in NCSI and agreed to treat patients who presented with acute myocardial infarction and cardiogenic shock using a standard protocol, which involved rapid initiation of mechanical circulatory support (MCS) with an Abiomed Impella 2.5 or Impella CP heart pump. This is done along with a right heart catheterization to place a pulmonary artery (PA) hemodynamic catheter to assess status of right and left ventricular heart function. The study enrolled 406 patients between July 2016 and December 2020.
“More than 400 patients from across the country were enrolled in this study, including a third of patients who had already suffered a cardiac arrest,” Basir said. “The survival rate of 71% is significantly higher than any other previous study on cardiogenic shock. The protocol standardizes the process of care provided by nurses, technicians, interventional cardiologists and critical care physicians, providing predictable care in high risk and complex patients. The protocol has already saved many lives and will continue to do so as more hospitals implement its principles.”
 The treatment algorithm, available at henryford.com/cardiogenicshock, emphasizes quick recognition of the condition, then inserting a temporary straw-sized pump into the heart to keep blood flowing throughout the body. The Impella heart pump, an FDA-approved device, is inserted through a catheter in the groin as soon as the patient arrives at the hospital. Doctors then treat the cause of the heart attack, either inserting a stent, removing a clot or taking other necessary action.
The treatment algorithm, available at henryford.com/cardiogenicshock, emphasizes quick recognition of the condition, then inserting a temporary straw-sized pump into the heart to keep blood flowing throughout the body. The Impella heart pump, an FDA-approved device, is inserted through a catheter in the groin as soon as the patient arrives at the hospital. Doctors then treat the cause of the heart attack, either inserting a stent, removing a clot or taking other necessary action.
"It is a big thing and will have a major impact for cardiology," explained Mohammad Zaidan, M.D., an interventional cardiologist at Henry Ford Hospital who was involved with the study and spoke during a hospital press conference on the study results. "Many of these patients would not have survived if it were not for this protocol."
Cardiogenic Shock Patient Classifications and Survival Rates
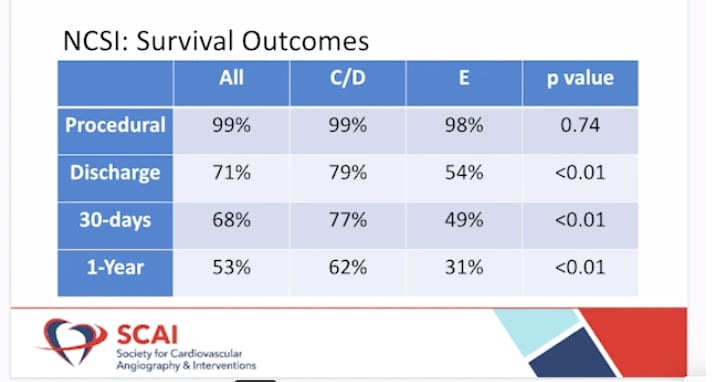
The study investigators categorized patients according to SCAI Shock classification stages. For SCAI stages C/D, they found higher survival to discharge (79%), 30-days (77%) and 1-year (62%) compared to historical data in similar patients during the last 30 years. SCAI stage E patients, who are on the brink of cardiovascular collapse, also had improved survival to discharge (54%), 30-days (49%), and 1-year (31%) compared to recent studies of patients who were not treated with the NCSI protocol that published in Journal of American College of Cardiology (33% survival to discharge) and Catherization and Cardiovascular Interventions (22.6% survival to 30-days).
The average patient age was 64 (±12 years), 24% were female and 67% were admitted in shock. Results found that early use of MCS and invasive hemodynamics is associated with an increased patient procedural survival (99%), survival to discharge (79%), survival to 30-days (77%), and survival to 1-year (62%) for patients presenting in stage C/D shock and 98%, 49%, 46%, 32% for patients in stage E shock (p=0.01).
To learn more about the NCSI, visit henryford.com/cardiogenicshock.
Next Steps to Improve Survival of Cardiogenic Shock Patients
Basir and O'Neill said they will be starting two more trials later this year to see if AMICS mortality can be further improved.
Basir said the ISOSHOCK trial will use the NCSI protocol with Impella and randomize patients with the addition of super saturation oxygenation therapy (SSO2), or without. This study will include 50 sites in the U.S. and additional sites in Canada and Europe. He said the goal is to see if this helps reduce infarct size and can reduce the incidence of heart failure. They hope to have results in three to four years.
Another randomized controlled trial, RECOVER IV, is being developed as a two-arm study to assess whether Impella pre-PCI is superior to PCI without Impella in AMICS patients. The co-principle investigators of this trial are O'Neill, Navin Kapur at Tufts Medical Center, and Gregg Stone at Mount Sinai in New York.
"This trial will definitively show the life-saving efficacy of mechanical circulatory support in these patients," O'Neill said.
In addition, the DanGer Shock trial is an ongoing trial in Denmark and Germany assessing primary outcome of death from all causes at six months with Impella CP, compared to standard of care.
Analysis of SCAI Shock Stages Consensus Statement
Another late-breaking study presentation at SCAI is a study of the SCAI cardiogenic shock stages consensus document, which confirms the accuracy of the shock classification. A 2019 joint SCAI consensus statement proposed a new classification system describing the stages of cardiogenic shock, from A to E, to standardize classification of the disease. To understand if the SCAI shock stages provide mortality risk classification, an analysis of studies in PubMed was conducted examining clinical outcomes. Researchers identified 14 manuscripts of more than 15,000 patients presenting with cardiogenic shock, cardiac arrest or those admitted to the cardiac intensive care unit. The studies examined seven separate definitions of the SCAI shock stages, and each study demonstrated a stepwise increase in short-term (in-hospital or 30-day) mortality with each higher SCAI shock stage.
Findings show mortality varying across shock stage (A, 1-5%; B, 0-34%; C, 11-54%; D, 24-68%; E, 42-77%) and increased with additional risk factors including the presence of CA, systemic inflammation, poor hemodynamics, worsening shock and older age.
“These findings confirm the efficacy of the SCAI shock stage classification, allowing physicians a staged approach to communicate with their colleagues and the broader heart team how sick a patient is in a very consistent way,” said lead researcher Jacob Jentzer, M.D., critical care specialist, Mayo Clinic. “Our analysis should enhance physician confidence in the protocol to appropriately identify high-risk and low-risk patients, ultimately helping tailor therapy based on level of shock to improve patient outcomes.”
Find more news from the SCAI 2021 virtual meeting
Related Cardiogenic Shock Content:
VIDEO: Final Results of the National Cardiogenic Shock Initiative Show 71 Percent Survival — Interview with William O’Neill, M.D.
New Approaches to Reduce Cardiogenic Shock Mortality
VIDEO: The Importance of Ventricular Unloading in AMI and Cardiogenic Shock — Interview with Nevin Kapur, M.D.
VIDEO: How to Reduce Cardiogenic Shock Mortality by 50 Percent — Interview with Babar Basir, D.O.
SCAI Releases New Consensus Document on Classification Stages of Cardiogenic Shock
VIDEO: Cardiogenic Shock Initiative Continues to Reduce Mortality by 50 Percent — Interview with William O’Neill, M.D.
10 Reasons Why it is Time to Learn More About Cardiogenic Shock — by Emmanouil S. Brilakis, M.D.
VIDEO: Overview of the National Cardiogenic Shock Initiative — Interview with William O’Neill, M.D.


 April 28, 2023
April 28, 2023