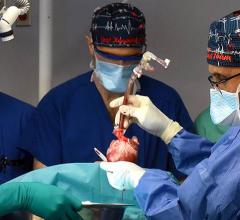
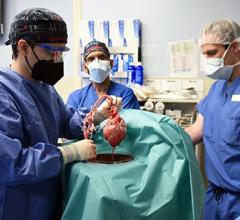
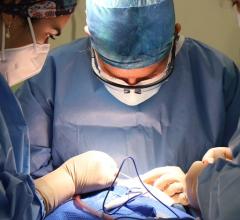
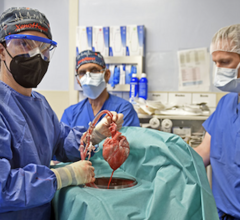
September 25, 2023 — After world’s first successful transplant in 2022, also performed at the University of Maryland Medical Center (UMMC), this groundbreaking transplant team performed second pig ...
ECMO Systems
Extracorporeal membrane oxygenation (ECMO) is used as a hemodymanic support system to increase the flow of blood in the body to allow the heart and lungs to rest in critically ill patients. It is similar to a heart-lung by-pass machine used in open-heart surgery. It pumps and oxygenates a patient's blood outside the body with blood flowing through tubing to an artificial lung in to add oxygen and remove carbon dioxide. The blood is then the blood is warmed to body temperature and pumped back into the patient’s body. VA ECMO is connected to both a vein and an artery when there are problems with the heart and lungs. VV ECMO is connected to one or more veins, usually near the heart, and is used when the problem in the lungs.
December 5, 2023 — Raymond Benza, MD, FACC, FAHA, FACP, a top expert in cardiovascular medicine and advanced heart ...
September 25, 2023 — After world’s first successful transplant in 2022, also performed at the University of Maryland ...
September 13, 2023 — The Smidt Heart Institute at Cedars-Sinai has earned a prestigious designation for its excellence ...
June 30, 2023 — A new study published in The Lancet has revealed the most extensive analysis to date on what led to the ...
June 2, 2023 — Dr. Philip Cardiff, Associate Professor at University College Dublin's School of Mechanical and Materials ...
November 30, 2022 — The Smidt Heart Institute at Cedars-Sinai has selected board-certified cardiothoracic surgeon Tyler ...
November 15, 2022 — Ten months after transplanting the first genetically-modified pig heart into a human patient ...
November 7, 2022 — The immediate use of veno-arterial mechanical circulatory extracorporeal membrane oxygenation (ECMO) ...
June 29, 2022 — While lower vertebrates can repair their adult hearts after a heart attack, mammals — including humans — ...

The University of Maryland Medical Center has announced that David Bennett, the 57-year old patient with terminal heart ...
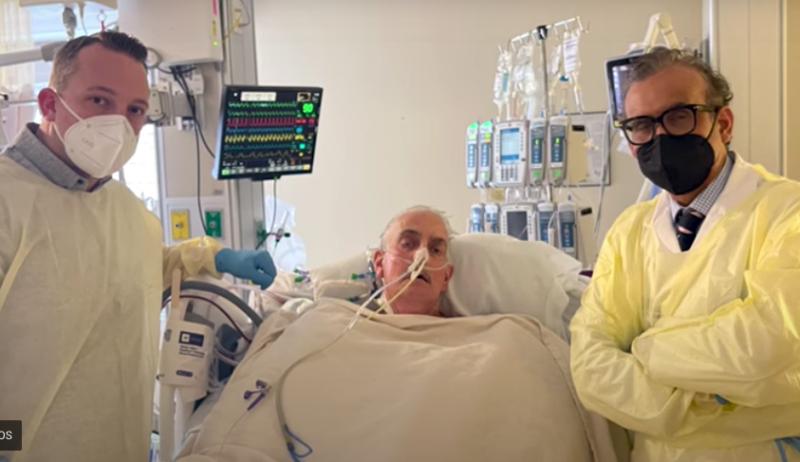
In January, The University of Maryland School of Medicine (UMSOM) and the University of Maryland Medical Center (UMMC) ...
February 8, 2022 — Extracorporeal membrane oxygenation (ECMO) is a lifesaving treatment for critically ill neonates ...

January 10, 2022 — The University of Maryland School of Medicine (UMSOM) and the University of Maryland Medical Center ...
December 26, 2021 — Here is a list of the most popular videos on the DAIC website from the past year in 2021 based on ...
December 7, 2021 – Critically ill patients with COVID-19 can safely be transported for lifesaving extracorporeal ...

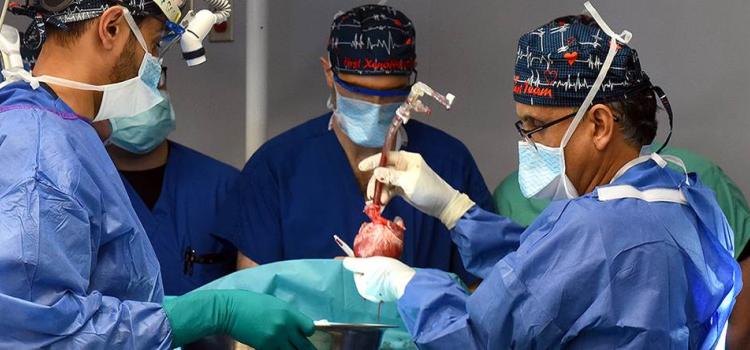
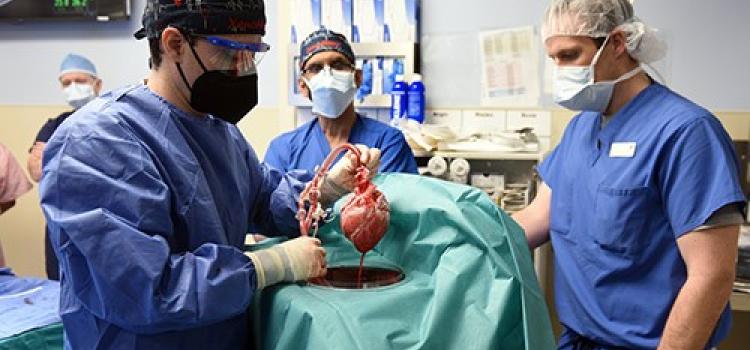


 December 05, 2023
December 05, 2023