October 26, 2020 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to Abiomed for an all-in-one, compact extracorporeal membrane oxygenation (ECMO) cardiopulmonary bypass system called the Abiomed Breethe OXY-1 System.
The ECMO system provides cardiopulmonary bypass support for patients whose lungs can no longer provide sufficient end organ oxygenation. The 510(k) clearance is to pump, oxygenate, and remove carbon dioxide from blood during cardiopulmonary bypass for up to six hours. The system can help provide oxygenation to patients suffering from cardiogenic shock or respiratory failure such as ARDS, H1N1, SARS, or COVID-19 (SARS-CoV-2). When used with the Impella heart pump it can unload the heart and oxygenate the body, a combination therapy known as ECpella.
Abiomed’s Breethe technology is a novel, easy-to-use cardiopulmonary bypass system that is designed for mobility. The components of the system are designed to reduce the overall equipment footprint, support patient ambulation, and provide an intuitive interface for health care providers to setup and manage. The integrated pump lung unit is engineered with volute spiral technology for uniform blood flow with minimal stagnation and advanced gas exchange technology that allows for full therapy with reduced oxygen requirements.
“Abiomed has a long-established track record of bringing to market improved options to support physicians with innovative technology like Breethe, which is designed to provide advanced respiratory and cardiac support,” said Bartley Griffith, M.D., the Hales Distinguished Professor of Surgery at University of Maryland, School of Medicine. “Abiomed is committed to advancing heart and lung therapies to help improve patient care and ultimately outcomes.”
“The clinical community has long been in need of innovation compared to traditional extracorporeal circulation therapy,” said Zachary Kon, M.D., cardiothoracic surgeon at New York University (NYU). “The Breethe system is a breakthrough technology because it supports transition from bed to ambulation via system portability. This system has the potential to revolutionize the way we think about extracorporeal life support therapy and can improve patient care.”
To help health care providers achieve the best possible outcomes, the Breethe system will be supported 24 hours a day, seven days a week by Abiomed’s experienced field-based, in-hospital clinical team and on-call team from the Clinical Support Center.
This ECMO technology adds to Abiomed’s innovative portfolio focused on native heart and lung recovery. For many patients in cardiogenic shock, Abiomed now provides the treatment options of both the catheter-based Impella heart pump, which unloads the left ventricle, perfuses end organs and allows the heart to rest and recover, and Breethe, which provides oxygenation. Impella and Breethe can work together as ECpella to unload the heart and oxygenate the body.
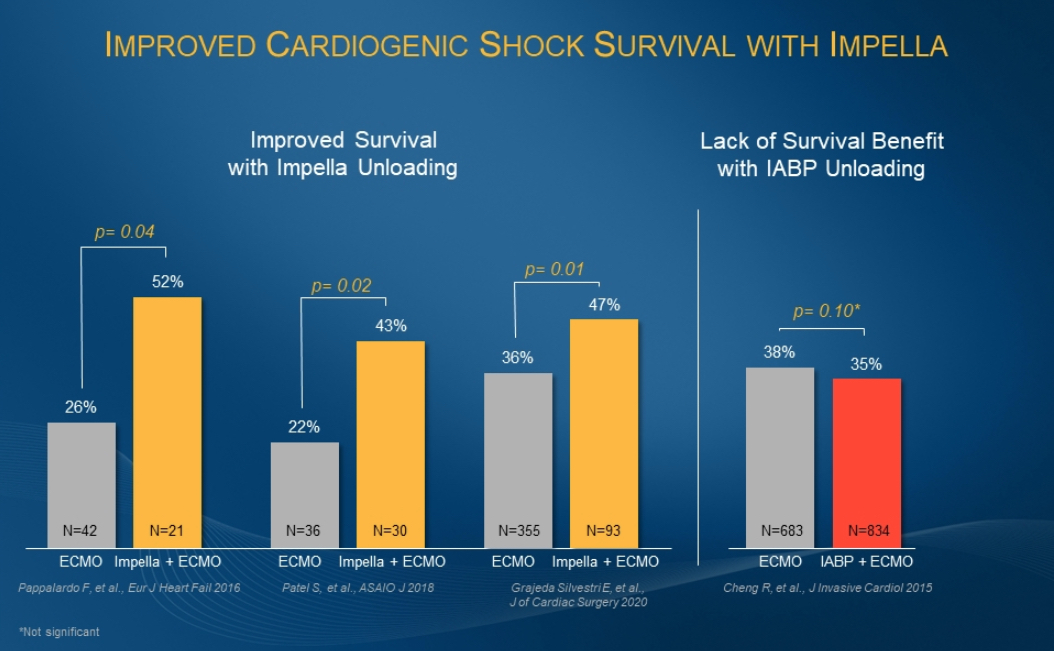
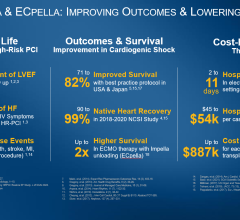
Multiple studies support the association of ECpella therapy to improve outcomes for patients who are suffering from cardiogenic shock and require oxygenation. A study published this month in Circulation examined data from 686 consecutive patients at 16 tertiary-care centers from four countries and found ECpella was associated with increased 30-day survival (43% vs 37%; p=0.03). The European Journal of Heart Failure, ASAIO, and the Journal of the American College of Cardiology have published studies that conclude use of ECpella was associated with increased survival rates, as compared to patients who were treated with ECMO only. This benefit is not seen in patients treated with ECMO combined with the intra-aortic balloon pump (IABP). (see figure 1) Additionally, the study in the European Journal of Heart Failure found higher rates of heart recovery with ECpella use than with ECMO only (62% vs 36%; p=0.048).
In August, the FDA issued an emergency use authorization (EUA) for all left-sided Impella heart pumps to provide left ventricular unloading and support to COVID-19 patients who are undergoing ECMO treatment and develop pulmonary edema or myocarditis.
Abiomed plans to have a controlled launch of the Breethe system at hospitals in the United States, with full U.S. commercial availability expected in calendar year 2021.
Details on the Abiomed Breethe OXY-1 System
The Abiomed Breethe OXY-1 System is cleared by the FDA to provide extracorporeal circulation. The OXY-1 System pumps, oxygenates and removes carbon dioxide from blood during cardiopulmonary bypass up to six hours in duration.
Under guidance issued by FDA, on April 6, 2020, the Abiomed Breethe OXY-1 System is now permitted to be used temporarily in the U.S. for ECMO therapy greater than six hours. Therefore, it now has a limited indication modification for use longer than six hours in an ECMO circuit to treat patients who are experiencing acute temporary respiratory or acute cardiopulmonary failure. This limited indication modification for ECMO therapy greater than six hours has not been cleared or approved by FDA and is in effect only for the duration of the public health emergency related to COVID-19 as declared by the U.S. Department of Health and Human Services (HHS).
“As a leader in technology and innovation, the Breethe system is a natural addition to Abiomed’s existing product portfolio,” said Michael R. Minogue, Abiomed’s chairman, president and chief executive officer. “This ECMO technology will allow us to support new patient populations, such as COVID-19 patients and others who need lung support, and provide combination ECpella therapy to Impella patients who need oxygenation. Furthermore, we will advance the field of native lung recovery and improve patient outcomes by collecting critical research data and developing and teaching best practices.”
For more information: www.abiomed.com
Related COVID-19 ECMO Support Content:
Abiomed Adds ECMO Cardiopulmonary Support to its Portfolio
FDA Allows Emergency Use of Impella for COVID-19 Patients on ECMO
VIDEO: ECMO Support Effective in Sickest COVID-19 Patients — Interview with Ryan Barbaro, M.D.,
FDA Approves ECMO to Treat COVID-19 Patients
ECMO Used to Treat Adult Respiratory Distress Syndrome Case
FDA Allows Emergency Use of Impella for COVID-19 Patients on ECMO
The Cardiovascular Impact of COVID-19


 April 28, 2023
April 28, 2023