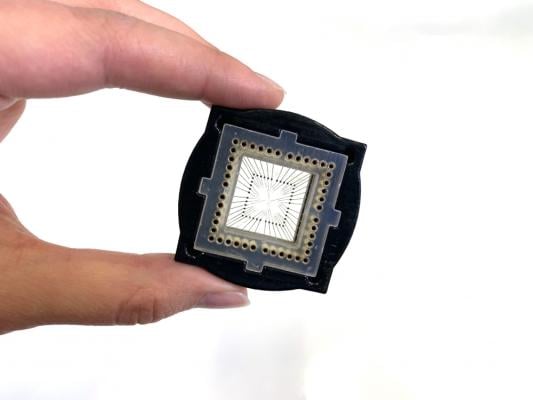
Photo courtesy of Tissue Dynamics
August 9, 2023 — A team of researchers, led by the Hebrew University of Jerusalem (HU), has developed a tiny human heart model that could potentially transform cardiovascular research and animal-free drug testing.
Cardiovascular diseases remain the leading cause of global mortality, underscoring the critical importance of this pioneering development. The study, published in Nature Biomedical Engineering, introduces a self-paced, multi-chambered human heart model, approximately half the size of a rice grain.
“Integrating our complex human heart model with sensors allowed us to monitor critical physiological parameters in real-time, revealing intricate mitochondrial dynamics that drive cardiac rhythms," said Prof. Yaakov "Koby" Nahmias, Director of the Hebrew University Alexander Grass Center for Bioengineering, and a Fellow of the American Institute for Medical and Biological Engineering (AIMBE). “This is a new chapter in human physiology."
Prof. Nahmias and his team created a highly accurate replica of a heart using human induced pluripotent stem cells (hiPSCs), which are derived from adult, non-reproductive cells that have been genetically reprogrammed. The tiny model has multiple chambers, pacemaker clusters, epicardial membrane, and endocardial lining, with all features meticulously designed to mimic a human heart’s structure and functionality.
The heart model detected a new form of cardiac arrhythmia, distinct from those observed in traditional animal models, which will enable researchers to gain invaluable insights into the precise effects of pharmaceutical compounds on the human heart.
One of the most significant features is the new model heart’s ability to provide real-time measurements of essential parameters such as oxygen consumption, extracellular field potential, and cardiac contraction. This capability enabled the scientists to gain unprecedented insights into heart function and diseases that could significantly advance the cardiovascular research field.
In the study, the researchers tested the heart model's response to the chemotherapeutic drug mitoxantrone, commonly used to treat leukemia and multiple sclerosis. Through these experiments, the researchers pinpointed how mitoxantrone induces arrhythmia by disrupting the heart's electro-mitochondrial coupling. The team also discovered a potential solution by administering metformin, which showed promise for mitigating the drug's adverse effects.
Partnering with Tissue Dynamics Ltd, a biotechnology company founded at Hebrew University by Prof. Nahmias, the scientists have developed a robotic system that can screen 20,000 tiny human hearts in parallel for drug discovery applications. This micro-physiological system has vast potential applications and is likely to accelerate the discovery of safer and more effective pharmaceutical interventions.
With this tiny heart model, pharmaceutical companies could make significant strides in developing safer, and more effective, medications for patients worldwide, improving patient outcomes and potentially saving lives.
"This miniature human heart model has the potential to reshape drug testing practices, advance our understanding of cardiovascular diseases, and ultimately contribute to a healthier and more sustainable future," said the researchers.
For more information: http://new.huji.ac.il/en
Related Cardiac Stem Cell Content:
Tiny Beating Hearts Created With Stem Cells at the University of Washington
What is New in Cardiology? A Review of All Major Emerging Technologies for Heart Diseases
Three Years After Stem Cell Trial for Heart Failure was Abandoned New Evidence Shows it Works
Stem Cell Therapy Improved Quality of Life in Patients with Microvascular Dysfunction
Placental Stem Cells Can Regenerate the Heart After Heart Attack
How Biotechnology is Impacting the Treatment and Prevention of Heart Disease
Heart Muscle Cells Created From Stem Cells Beat in Sync
FDA Releases Final Guidances on Regenerative Medicine Therapies


 January 15, 2026
January 15, 2026 









