November 23, 2021 — The antiplatelet drug class of P2Y12 inhibitors that block the activity of platelets and prevents clotting, failed to extend survival or lessen disease severity in patients with ...
Antiplatelet and Anticoagulation Therapies
This channel includes news and new technology innovations for cardiovascular pharmaceutics. This includes antiplatelet agents, anticoagulation drugs, international normalized ratio (INR) testing, oral anticoagulants, IV administered drugs such as Heprin, and dual antiplatelet therapy (DAPT). The most commonly used anticoagulant is warfarin, which available in generic form at a low cost. However, it has a narrow therapeutic window and its effectiveness is altered by food containing vitamin K. To regulate warfarin, regular INR testing is needed. The newer anticoagulation drugs are referred to as novel oral anticoagulant (NOAC). However, since some of these drugs are now more than six years old, they are commonly referred to as non-vitamin K oral anticoagulant (NOAC), and the newest term, direct oral anticoagulant (DOAC). NOAC or DOAC agents have much larger therapeutic windows, do not require INR testing. Aspirin and clopidogrel (Plavix) are the most commonly used antiplatelet agents for the prevention of heart attacks and stroke. They are often prescribed together as DAPT.
August 30, 2023 — BIOFLOW-DAPT one-year-data demonstrated non-inferiority and a good safety profile for the Orsiro ...
August 28, 2023 — Prasugrel monotherapy after percutaneous coronary intervention (PCI) with drug-eluting stents is not ...
June 1, 2023 — New insights from the TWILIGHT trial showed that ticagrelor monotherapy after three months of ticagrelor ...
March 24, 2023 — According to the U.S. Food and Drug Administration (FDA), Ascend Laboratories LLC is voluntarily ...
November 14, 2022 — Bivalirudin is a safer and more effective anticoagulant than heparin for treating patients with the ...
November 23, 2021 — The antiplatelet drug class of P2Y12 inhibitors that block the activity of platelets and prevents ...
November 22, 2021 — Fewer than 10 days of low-molecular-weight heparin (LMWH) after stenting for extensive iliofemoral ...
November 18, 2021 — People who are on the antiplatelet medication ticagrelor and need coronary artery bypass surgery may ...
November 17, 2021 — Taking daily low-dose aspirin for seven years did not affect the risk of dementia or mental decline ...
November 9, 2021 — Utilizing a magnetically-controlled capsule endoscopy system, the double-blind, randomized OPT-PEACE ...

There has been debate in recent years over balancing the risks vs. benefits of aspirin for the prevention of heart ...
October 4, 2021 — One month of dual antiplatelet therapy (DAPT) following stent implantation in high bleeding risk ...
September 8, 2021 — Continuous heart rhythm monitoring – with anticoagulation if atrial fibrillation is detected – does ...
September 1, 2021 — The STOPDAPT-2 ACS trial does not support the use of one month of dual antiplatelet therapy (DAPT) ...
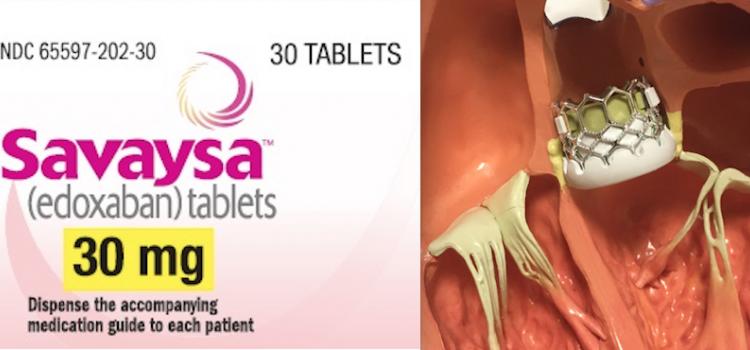
September 1, 2021 – The anticoagulant edoxaban (Savaysa) may be just as effective as warfarin for preventing heart ...




 August 30, 2023
August 30, 2023