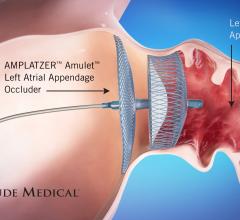
March 29, 2020 — Patients with atrial fibrillation (AF) who took oral anticoagulants alone after undergoing ...
Antiplatelet and Anticoagulation Therapies
This channel includes news and new technology innovations for cardiovascular pharmaceutics. This includes antiplatelet agents, anticoagulation drugs, international normalized ratio (INR) testing, oral anticoagulants, IV administered drugs such as Heprin, and dual antiplatelet therapy (DAPT). The most commonly used anticoagulant is warfarin, which available in generic form at a low cost. However, it has a narrow therapeutic window and its effectiveness is altered by food containing vitamin K. To regulate warfarin, regular INR testing is needed. The newer anticoagulation drugs are referred to as novel oral anticoagulant (NOAC). However, since some of these drugs are now more than six years old, they are commonly referred to as non-vitamin K oral anticoagulant (NOAC), and the newest term, direct oral anticoagulant (DOAC). NOAC or DOAC agents have much larger therapeutic windows, do not require INR testing. Aspirin and clopidogrel (Plavix) are the most commonly used antiplatelet agents for the prevention of heart attacks and stroke. They are often prescribed together as DAPT.
March 28, 2020 — The TAILOR-PCI trial that used genetic testing to guide which antiplatelet medication was given to ...
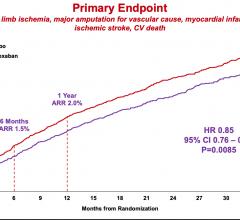
March 28, 2020 — People with symptomatic peripheral artery disease (PAD) who took the blood thinner rivaroxaban with ...
Carey Kimmelstiel, M.D., FACP, FACC, director, interventional cardiology, director, cardiac catheterization lab, Tufts ...
February 3, 2020 — The U.S. Food and Drug Administration (FDA) has approved a the CATALYST trial designed to assess ...

December 27, 2019 — The U.S. Food and Drug Administration (FDA) has approved two applications for the first generics of ...
December 12, 2019 — Low-dose aspirin was not associated with a reduced risk of a fatal heart attack among African ...
The latest in interventional cardiology clinical data and new device technologies were highlighted at the annual Transca ...
Roxana Mehran, M.D., FACC, FACP, FCCP, FESC, FAHA, FSCAI, professor of medicine and director of interventional ...
Ajay J. Kirtane, M.D., associate professor of medicine at Columbia University Irving Medical Center and director of the ...
Roxana Mehran, M.D., FACC, FACP, FCCP, FESC, FAHA, FSCAI, professor of medicine and director of interventional ...
American Heart Association President Robert Harrington, M.D., interventional cardiologist and the Arthur L. Bloomfield ...
September 30, 2019 – The first randomized trial to compare a durable polymer drug-eluting stent to a polymer-free drug ...
September 30, 2019 — A biodegradable polymer everolimus-eluting stent (BP-EES) followed by four months of dual ...
September 30, 2019 – Data from the EVOLVE Short DAPT study found that shortened three-month dual antiplatelet therapy ...

 March 29, 2020
March 29, 2020