September 6, 2023 — The Cardiovascular Research Foundation (CRF) has announced the TCT 2023 late-breaking clinical trials. TCT is the annual scientific symposium of CRF and the world’s premier ...
Stents Drug Eluting
This channel includes news and new technology innovations for drug eluting stents (DES). These drug coated stents were developed to solve a frequent problem with bare metal stents, which can cause neointimal hyperplasia (scar tissue growth) in some patients. The antiproliferative drugs used on DES prevent the growth of tissue. One downside of DES is the requirement for patients to take long-term antiplatelet therapy to prevent the possible formation of clots on these stents. Newer generation DES use technologies help the vessels heal faster, which may allow reduce the duration of dual antiplatelet therapy (DAPT), or use a single drug, usually eliminating aspirin. This section includes news for both metallic and bioresorbable drug-eluting stents and related clinical trial data.
July 11, 2024 — Medical device company R3 Vascular Inc., developer of novel, best-in-class bioresorbable scaffolds for ...
July 2, 2024 — Biotronik announced the availability of an expanded Maximum Allowed Diameters (MAD) range for the Orsiro ...
June 3, 2024 — Elixir Medical, a developer of transformative technologies to treat cardiovascular and peripheral disease ...
October 31, 2023 — SMT (Sahajanand Medical Technologies), a leading medical device company in India, focused on ...
October 10, 2023 — Elixir Medical, a developer of innovative cardiovascular technologies, announced it will present ...
September 6, 2023 — The Cardiovascular Research Foundation (CRF) has announced the TCT 2023 late-breaking clinical ...
August 30, 2023 — BIOFLOW-DAPT one-year-data demonstrated non-inferiority and a good safety profile for the Orsiro ...
July 13, 2023 — Elixir Medical, a developer of breakthrough cardiovascular technologies, announced enrollment completion ...
July 12, 2023 — In a late breaking trial session during EuroPCR 2023 in Paris, on behalf of the HOST-IDEA study ...
May 25, 2023 — First-generation bioresorbable vascular scaffolds (BVS) may be just as effective as drug-eluting metallic ...
May 17, 2023 — Elixir Medical has reported that important data were presented at a late-breaking clinical session during ...
January 9, 2023 — When we were little, our parents told us to take our vitamins so we could grow big and strong. Now ...
January 2, 2023 — The U.S. Food and Drug Administration (FDA) has approved the BioFreedom Drug Coated Coronary Stent (DC ...
December 23, 2022 — According to Coherent Market Insights, the global Drug Eluting Stents market is estimated to be ...

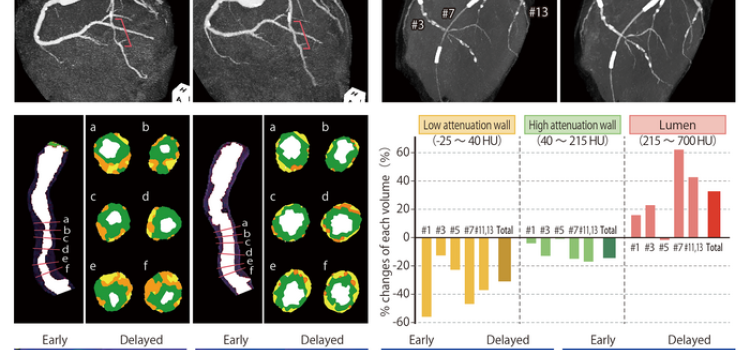

 July 11, 2024
July 11, 2024