November 24, 2020 — One-year results off the PIONEER III study comparing the safety and efficacy of the Supreme HT (Healing-Targeted) drug-eluting stent (DES), to the Xience or Promus Durable Polymer ...
Stents Bioresorbable
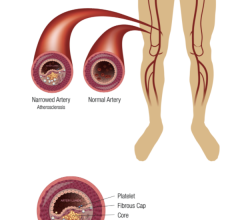
This channel includes news and new technology innovations for bioresorbable stents (BRS). These devices are also referred to as bioabsorbable stents, bioresorbable scaffolds and dissolving stents. BRS are designed as an alternative to permanent metallic stent implants, which cause issues in a small number of patients with in-stent restenosis, late-stent thrombosis and require use of long-term antiplatelet therapy. Metallic stents also cause issues with CT and MRI imaging and may prevent future options for coronary bypass graft (CABG) surgery. BRS are supposed to remove avoid these issues by dissolving and disappearing from the vessel after a period of 2-4 years. This, returns the vessel to its natural state and allows for the return of vasodilatation and vasoconstriction. BRS have had some issues in clinical trials not being able to match the performance of standard metallic drug eluting stents (DES) because of their thick stent struts. Newer generation BRS are in development with struts smaller than 100 micros, with will be closer to those of current generation metallic stents
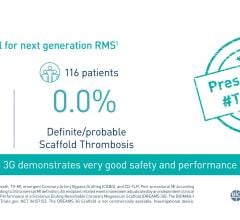
May 30, 2024 — Biotronik announced the presentation of the 12-month results from the BIONETIC-I study this week at LINC ...
February 14, 2024 — Efemoral Medical, developer of advanced interventional bioresorbable therapies, today announced that ...
October 30, 2023 — Abbott announced late-breaking data from the LIFE-BTK clinical trial evaluating the Esprit BTK ...
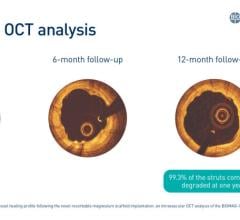
September 5, 2023 — New data from the BIOMAG-I first-in-human trial shed light on the vascular healing process following ...
March 16, 2023 — Prof. Michael Haude, BIOMAG-I Coordinating Clinical Investigator, presented the latest results of the B ...
October 11, 2022 — Efemoral Medical, developer of advanced interventional bioresorbable therapies, announced that it ...
September 28, 2022 — In the "TCT Innovation" session Prof. Michael Haude, BIOMAG-I Coordinating Clinical Investigator ...
September 27, 2022 — BIOTRONIK announced the presentation of new full-cohort 2-year BIOSOLVE-IV data. In a poster ...
December 16, 2020 — Efemoral Medical announced the first-in-human (FIH) use of the its Efemoral bioresorbable vascular ...
November 24, 2020 — One-year results off the PIONEER III study comparing the safety and efficacy of the Supreme HT ...
Here are some of the key takeaways from the late-breaking interventional cardiology and structural heart trials ...
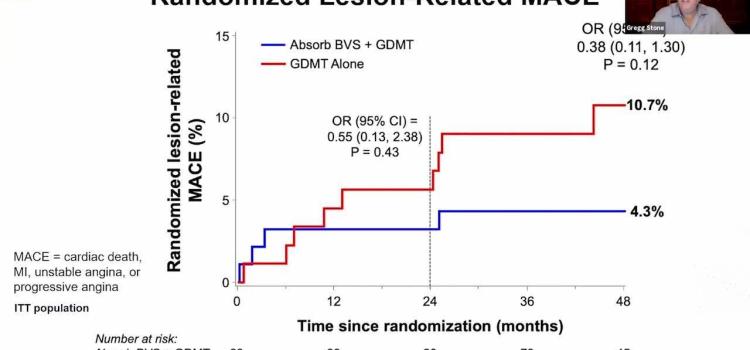
Gregg Stone, M.D., presents the results of the PROSPECT ABSORB Trial in a press conference at the 2020 ranscatheter ...
September 3, 2020 — Abbott today announced the start of the LIFE-BTK clinical trial to evaluate the safety and ...

Cardiovascular diseases (CVDs) are among the leading causes of death across the globe. For patients suffering from high ...
November 8, 2019 — The Absorb bioresorbable stent (BVS) demonstrated good patency and clinical outcomes in patients with ...



 May 30, 2024
May 30, 2024