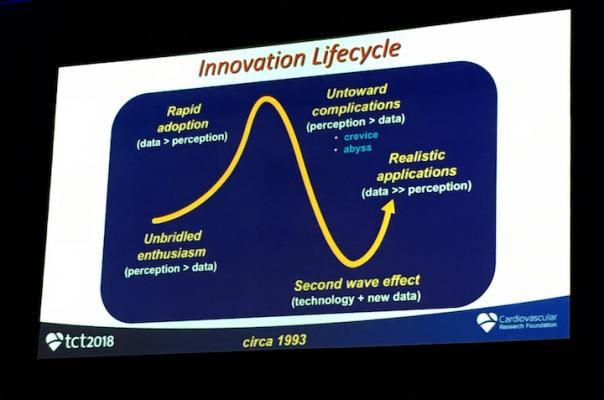
The cardiovascular innovation cycle, presented by TCT Course Director Martin Leon, M.D., at TCT 2018.
There is a hype cycle surrounding new technologies in all industries, but medicine is unique because of its focus on clinical trial data as one of the key determining factors of whether a new device becomes standard of care or dies on the vine. Here is a great summary of this cycle with cardiovascular devices.
Over the past decade of covering the Transcatheter Cardiovascular Therapeutics (TCT) meetings, I have seen an interesting slide on the innovation cycle for interventional cardiology device technologies presented numerous times by Course Director Martin B. Leon, M.D., director of the Center for Interventional Vascular Therapy and professor of medicine, NewYork-Presbyterian, Columbia University Medical Center. The slide is always the same since he first presented it at TCT in 1993, but it holds universally true today in 2018.
Leon said the cycle starts with the introduction of a new technology that garners unbridled enthusiasm because of the promise the technology holds to solve problems with the current standard of care. The early data seems great and when it enters the market, there is rapid adoption as everyone wants to try to use it. However, there are inevitably some complications that are discovered once the technology is used on a large scale, either due to implant, operator skill or previously unknown complications. When data on the complications are presented, there is a steep drop-off in the use of the technology.
At this point in the cycle, Leon said it is important to refine the technology to overcome the issues presented by the first-generation device. Once that is achieved and clinical data confirms the issues are resolved, there is a second wave of adoption for the technology, as cardiologists are cautiously optimistic about using the improved technology. He said that crash where the enthusiasm dissolves because of cold, hard data is when the real science of refining the technology into something better can take place.
Certainly this cycle mirrored the case with drug-eluting stents and the discovery of polymer-related late-stent thrombosis. The same is true with renal denervation and bioresorbable stents.
At TCT 2018, Leon used this slide in sessions to describe where interventional cardiology is at today with bioresorbable stent technology — at the bottom of the abyss. However, he said there are new bioresorbable technologies still being developed that already hold promise to resolve the issues inherent with the first-generation Absorb stent. This includes new bioresorbable scaffolds (BRS) that use different polymers to possibly avoid late thrombosis, and devices with the same strut thickness of today's metallic stents to make the devices easier to deliver and allow use in smaller vessels.
Two years ago, all TCT sessions on bioresorbable technologies were packed and it seemed as if metallic stents had no long-term future. What a difference a year makes from TCT 2017, when the poor ABSORB III Trial data was presented and Abbott pulled the Absorb off the market. At TCT 2018, bioresorbable technology definitely took a back seat to metallic stent presentations, where the industry has definitely refocused its interest.
"I want to be a wild enthusiast, not a doubting skeptic," Leon said about BRS technology. "The Absorb was not easy to use and there was a safety concern. The safety signal is really what distracted people's attention with bioresorbable stents. We are not going to have an avalanche of new bioresorbable stents, but there will be a few second-generation stents that have overcome the limitations of the first-generation stent."
However, the new clinical evidence will have to be persuasive enough with these new BRS devices to convince him and the rest of the interventional cardiology community that the technology is truly ready for prime time.
Read the article from TCT 2018 "The Next Step in Bioresorbable Stent Technologies."
Watch an interview with Patrick Serruys, M.D., about the future of BVS technology.



 November 14, 2025
November 14, 2025 









