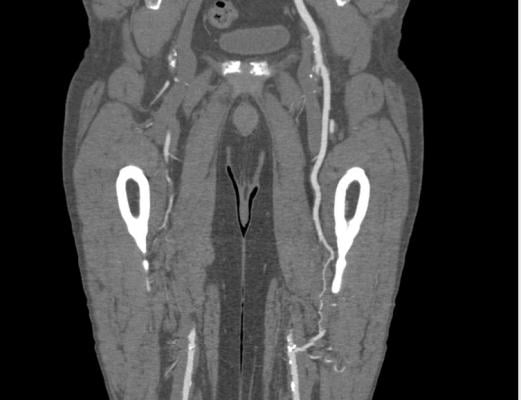
A peripheral artery disease (PAD) patient CT scan showing blockages in the femoral arteries in the legs with collateral flow in the leg on the right.
December 16, 2020 — Efemoral Medical announced the first-in-human (FIH) use of the its Efemoral bioresorbable vascular scaffold system (EVSS) with FlexStep Technology in the EFEMORAL I FIH clinical study. The bioresorbable stent is designed to address a broad range of patients with peripheral arterial disease (PAD), to alleviate symptoms and avoid reintervention for this historically challenging patient population.
The EVSS with FlexStep Technology was specifically developed to address the anatomical challenges and complex biomechanics of patients with symptomatic PAD. The patented FlexStep Technology, through the use of inter-scaffold spaces, combines flexibility with support to accommodate tortuosity and skeletal movement, while the balloon-expandable deployment system easily opens vessels and sustains healthy blood flow. The novel bioresorbable scaffold with long-term sirolimus elution aims to deliver therapeutic benefits across all lesion lengths and morphologies, prevent restenosis, and maintain patency while leaving no permanent implant behind.
"I am pleased to enroll the first patient in the EFEMORAL I study," said Dr. Andrew Holden, principal investigator and director of interventional radiology at Auckland City Hospital in New Zealand. "The treatment of peripheral arterial disease remains challenging as current therapies are often only temporarily effective. The system was easy to use and its unique design allows the artery to bend freely. This device has the potential to be the first safe and effective bioresorbable stent for femoro-popliteal disease."
PAD, also known as "poor circulation" or "hardening of the arteries," is a global plague. Worldwide, it affects approximately 202 million people,[1] including over 8.5 million people in the United States.[2] If left untreated, PAD can lead to severe disability and extremity amputation. Effectiveness of current interventional treatment remains limited with up to 50% of conventional endovascular procedures complicated by failure or recurrence within the first year.[3]
"I would like to thank Dr. Holden and the entire team for their efforts and collaboration in achieving this significant milestone," said Christopher Haig, co-founder and CEO of Efemoral Medical. "Efemoral celebrates our next step as a clinical-stage company, and while still early in the development process, we are excited about the potential of our technology to offer a durable clinical solution to patients and physicians."
Efemoral Medical is developing next-generation bioresorbable solutions to treat patients with vascular disease. The company's initial product, the Efemoral Vascular Scaffold System (EVSS) with FlexStep Technology, is designed to offer a dedicated strategy for PAD interventions. The Efemoral Vascular Scaffold System (EVSS) is an an investigational device outside the U.S.
For more information: efemoralmedical.com
Find more news on bioresorbable stents
References:
1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6377796/
2. https://www.heart.org/en/health-topics/peripheral-artery-disease/pad-toolkit
3. https://www.researchgate.net/publication/260118801_Nitinol_Self-Expanding_Stents_vs_Balloon_Angioplasty_for_Very_Long_Femoropopliteal_Lesions


 January 05, 2026
January 05, 2026 









