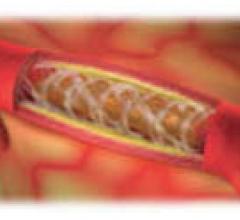
May 13, 2014 — Elixir Medical Corp. received European CE mark approval for its DESolve 100 Novolimus-eluting Bioresorbab ...
Stents Bioresorbable
This channel includes news and new technology innovations for bioresorbable stents (BRS). These devices are also referred to as bioabsorbable stents, bioresorbable scaffolds and dissolving stents. BRS are designed as an alternative to permanent metallic stent implants, which cause issues in a small number of patients with in-stent restenosis, late-stent thrombosis and require use of long-term antiplatelet therapy. Metallic stents also cause issues with CT and MRI imaging and may prevent future options for coronary bypass graft (CABG) surgery. BRS are supposed to remove avoid these issues by dissolving and disappearing from the vessel after a period of 2-4 years. This, returns the vessel to its natural state and allows for the return of vasodilatation and vasoconstriction. BRS have had some issues in clinical trials not being able to match the performance of standard metallic drug eluting stents (DES) because of their thick stent struts. Newer generation BRS are in development with struts smaller than 100 micros, with will be closer to those of current generation metallic stents
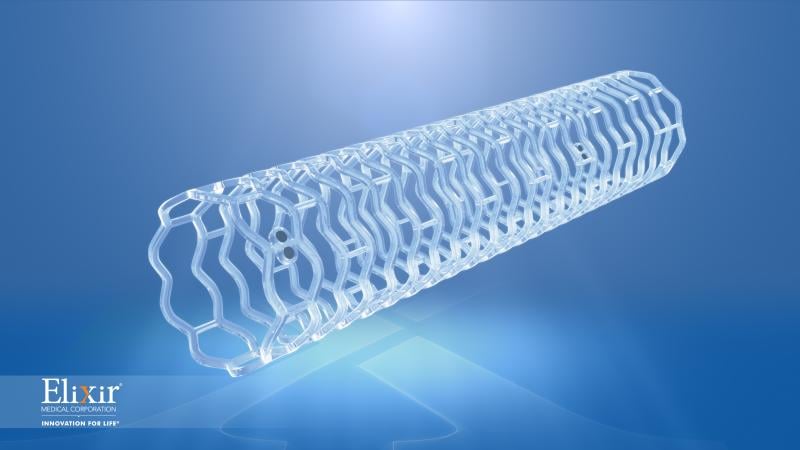
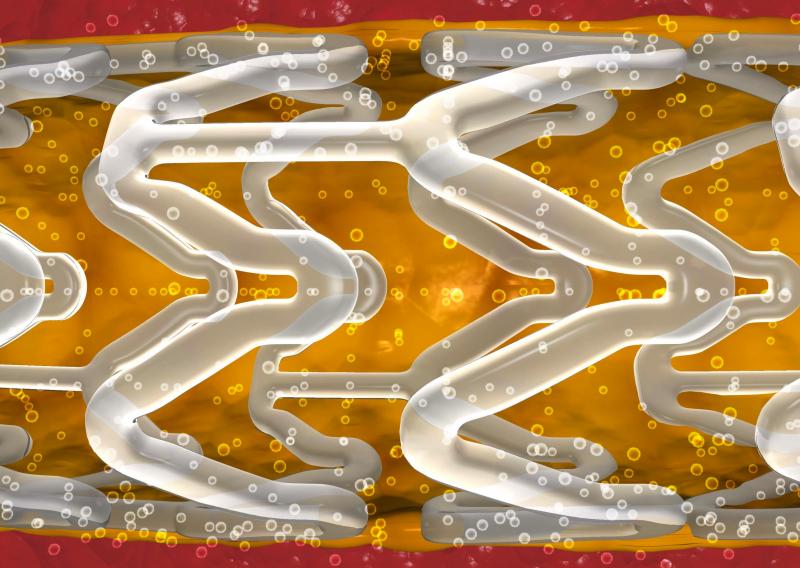
April 16, 2014 — Abbott announced it has completed enrollment of three clinical trials to support approvals of the ...
Bioresorbable stent technology was one of the big interventional technologies discussed at the American College of ...

March 31, 2014 — Privately held Arterial Remodeling Technologies (ART) signed a structured buyout agreement with Terumo ...
March 12, 2014 — Micell Technologies Inc. announced that imaging and clinical results from the DESSOLVE I and DESSOLVE ...
January 23, 2014 – Six-month results of the ESPRIT trial suggest a bioresorbable drug-eluting scaffold is effective in ...
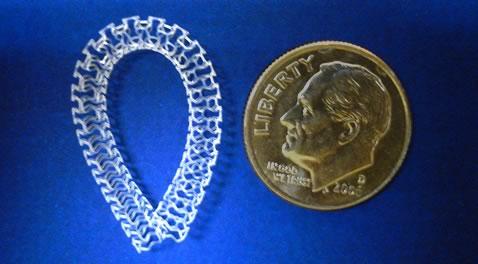

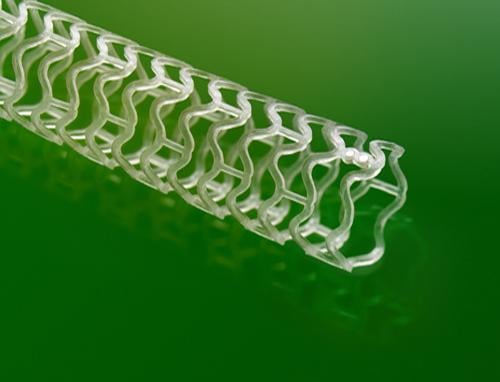
October 23, 2013 — Vascular Interventional Advances (VIVA) Physicians, a not-for-profit organization dedicated to ...
October 8, 2013 — Micell Technologies Inc. announced that a peer reviewed article discussing imaging and clinical ...

 May 13, 2014
May 13, 2014