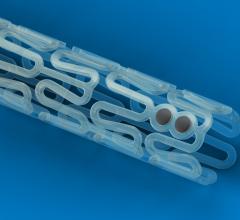
October 31, 2017 – Thirty-day results from ABSORB IV, the largest randomized everolimus-eluting bioresorbable vascular ...
Stents Bioresorbable
This channel includes news and new technology innovations for bioresorbable stents (BRS). These devices are also referred to as bioabsorbable stents, bioresorbable scaffolds and dissolving stents. BRS are designed as an alternative to permanent metallic stent implants, which cause issues in a small number of patients with in-stent restenosis, late-stent thrombosis and require use of long-term antiplatelet therapy. Metallic stents also cause issues with CT and MRI imaging and may prevent future options for coronary bypass graft (CABG) surgery. BRS are supposed to remove avoid these issues by dissolving and disappearing from the vessel after a period of 2-4 years. This, returns the vessel to its natural state and allows for the return of vasodilatation and vasoconstriction. BRS have had some issues in clinical trials not being able to match the performance of standard metallic drug eluting stents (DES) because of their thick stent struts. Newer generation BRS are in development with struts smaller than 100 micros, with will be closer to those of current generation metallic stents
October 31, 2017 — The U.S. Food and Drug Administration (FDA) issued a warning to healthcare providers that interim ...
October 25, 2017 — Amaranth Medical will provide an update at the annual Transcatheter Cardiovascular Therapeutics (TCT) ...

September 8, 2017 — Abbott Vascular has announced it will end commercial sales of its Absorb bioresorbable vascular ...
Stephen Ellis, M.D., professor of medicine and director of interventional cardiology at Cleveland Clinic, discusses the ...
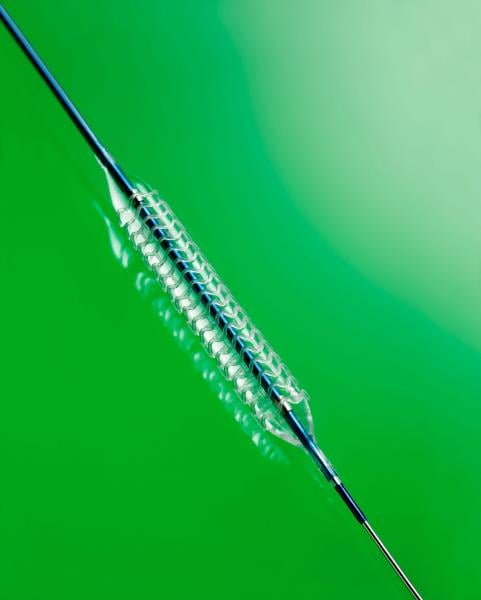
March 21, 2017 — In the late-breaking ABSORB III Trial two year results presented at the American College of Cardiology ...
Stents were the key focus of interventional cardiology for more than 20 years, but the focus has changed in recent years ...
DAIC has worked hard to bring useful technology news and resources to our readers. While we conduct reader surveys and ...

One of the big advancements in drug-eluting stent (DES) technology has been the development of bioresorbable polymers ...
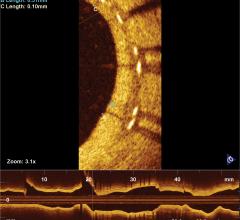
January 13, 2017 — Results from TRANSFORM-OCT, a prospective, randomized trial using optical coherence tomography (OCT) ...

The Diagnostic and Interventional Cardiology (DAIC) website had a record year in 2016, breaking 1 million page views for ...
November 9, 2016 – Results from a randomized, multicenter trial failed to show non-inferiority of hybrid, ultra-thin ...
Juan Granada, M.D., executive director and chief scientific officer of the Cardiovascular Research Foundation's Skirball ...
November 7, 2016 – Results from TRANSFORM-OCT, a prospective, randomized trial using optical coherence tomography (OCT) ...
November 7, 2016 — The 28th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium, Oct. 29-Nov. 3 ...

 October 31, 2017
October 31, 2017