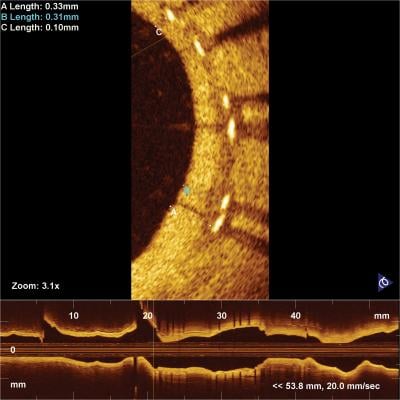
OCT imaging showing neointimal tissue coverage of metallic stent struts.
November 7, 2016 – Results from TRANSFORM-OCT, a prospective, randomized trial using optical coherence tomography (OCT) to evaluate strut coverage and neoatherosclerosis (NA), found that bioresorbable polymer-based drug-eluting stents (BP-EES) are comparable to durable polymer-based drug-eluting stents (DP-ZES). The trial examined the Boston Scientific Synergy stent and the Medtronic Resolute Integrity.
Findings were reported at the 28th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium.
“In this head-to-head in-vivo comparison of early and late healing response, the bioresorbable abluminal polymer Synergy everolimus-eluting stent was non-inferior at three-month and similar at 18-month follow-up to the durable conformal polymer Resolute zotarolimus-eluting stent,” said Giulio Guagliumi, M.D. with Ospedale Giovanni XXIII in Bergamo, Italy. “TRANSFORM-OCT adds a novel mechanistic dimension to the assessment of new-generation drug-eluting stents, consolidating the understanding that well designed and biocompatible polymers, regardless of whether they are durable or biodegradable, may favorably impact the long-term vascular response of these stents.”
To mitigate the risk related to durable polymer (DP), bioabsorbable polymers (BP) that coat only the abluminal side of the stent, avoiding contact with circulating blood, and render stent surface similar to those of BMS after complete absorption, have been developed. Whether BP abluminal coating may counteract in-vivo delayed healing and NA development in current thin-strut DES is unknown. TRANSFORM-OCT randomized 90 patients with multivessel disease (1:1) to a BP everolimus-eluting stent (BP-EES, Synergy) or a DP zotarolimus-eluting stent (DP-ZES, Resolute Integrity).
The primary endpoints were maximum length of consecutive frames with uncovered struts at three months (powered for non-inferiority of BP-EES) and the percentage of patients presenting with frames of NA at 18 months (powered for superiority of BP-EES) as measured by OCT. The three-month median percentage of covered struts was 79.1 (IQR 60.4, 89.8) for BP-EES and 78.4 (IQR 62.1, 87.8) for DP-ZES, P=0.93. The 18-month median percentage of covered struts was 99.4 (IQR 96.6-100) for BP-EES and 98.0 (IQR 94.4-99.8) for DP-ZES, P=0.14. The co-primary endpoint of in-stent NA at 18 months from 98.9% of all eligible patients was 11.6 % for BP-EES versus 15.9% in DP-ZES (P=0.59) with low percentage of frames with NA in both stent types (1.1 ± 3.1 for BP-EES versus 2.5 ± 9.1 for DP-ZES, P=0.33).
The TRANSFORM-OCT trial was designed, promoted and implemented by Ospedale Papa Giovanni XXIII, Bergamo, Italy, with unrestricted financial support provided by Boston Scientific International. The company was not involved with any of the study processes, including site selection, data collection, analysis, and drafting of the present abstract. Guagliumi reported consultant agreements with Boston Scientific and St. Jude Medical and receive research grants through the hospital by Abbott Vascular, St Jude Medical and Boston Scientific.
Three TCT 2016 Trials Failed To Show Differences in Patient Outcomes With Bioreorbable Polymers
There were three trials presented at TCT 2016 that compared metallic stents with bioresorbable polymers (including the Biosensor's Osiro and the Boston Scientific Synergy) to durable polymer stents (including the Medtronic Endeavor and Abbott Xience V). The data from thee trials all showed there was no statistical difference between bioresorbable and non-bioresorbable polymer metallic stents. There were high hopes that bioresorbable polymer metallic DES would be a big step forward to further reduce rates of late stent thrombosis and in-stent restenosis.
Read the related articles:
Bioresorbable Polymer Metallic Stents Did Not Improve Outcomes Compared to Durable Polymer Stents
For more information: www.crf.org


 November 14, 2025
November 14, 2025 









