November 7, 2019 — Although the Abbott Absorb fully bioresorbable drug-eluting stent (DES) was taken off the market due ...
Stents Bioresorbable
This channel includes news and new technology innovations for bioresorbable stents (BRS). These devices are also referred to as bioabsorbable stents, bioresorbable scaffolds and dissolving stents. BRS are designed as an alternative to permanent metallic stent implants, which cause issues in a small number of patients with in-stent restenosis, late-stent thrombosis and require use of long-term antiplatelet therapy. Metallic stents also cause issues with CT and MRI imaging and may prevent future options for coronary bypass graft (CABG) surgery. BRS are supposed to remove avoid these issues by dissolving and disappearing from the vessel after a period of 2-4 years. This, returns the vessel to its natural state and allows for the return of vasodilatation and vasoconstriction. BRS have had some issues in clinical trials not being able to match the performance of standard metallic drug eluting stents (DES) because of their thick stent struts. Newer generation BRS are in development with struts smaller than 100 micros, with will be closer to those of current generation metallic stents
August 21, 2019 — Increasing incidence of cardiovascular diseases worldwide is creating growth opportunities for the ...
There is a hype cycle surrounding new technologies in all industries, but medicine is unique because of its focus on ...
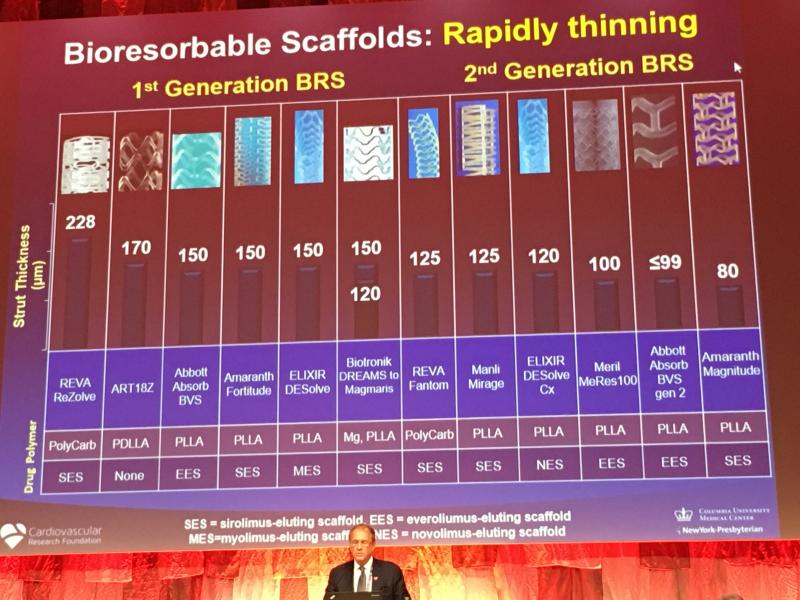
Bioresorbable stent (BRS) technology is not dead, but the unbridled enthusiasm seen two years ago for the technology has ...
October 12, 2018 – Reva Medical presented four key data sets demonstrating the capabilities of the company’s Fantom bior ...
A discussion with Professor Ian Meredith, MBBS, Ph.D., global chief medical officer and executive vice president, Boston ...
DAIC Editor Dave Fornell takes a tour of some of the most innovative new cardiovascular technology he found on the expo ...
October 1, 2018 — New results from the RENASCENT studies presented at the Transcatheter Cardiovascular Therapeutics (TCT ...
Patrick Serruys, M.D., Ph.D., Imperial College London, explains where development of bioresorbable scaffolds stands in ...

June 19, 2018 – Amaranth Medical offered new details about its 85-micron Defiance bioresorbable scaffold (BRS) recently ...
May 31, 2018 – Late-breaking trial results presented at the EuroPCR Congress, May 21-24 in Paris, France, found the ...
January 25, 2018 – Elixir Medical Corporation, a leader in the development of breakthrough adaptive remodeling ...
There was no shortage of interest in bioresorbable stent technologies at the many sessions offered on this topic or ...

November 8, 2017 – New results from the HARMONEE Japan/U.S. Registration Trial, reported by in a first report ...
A discussion with Ajay Kirtane, M.D., SM, director of the cardiac catheterization laboratories at New York-Presbyterian ...


 November 07, 2019
November 07, 2019









