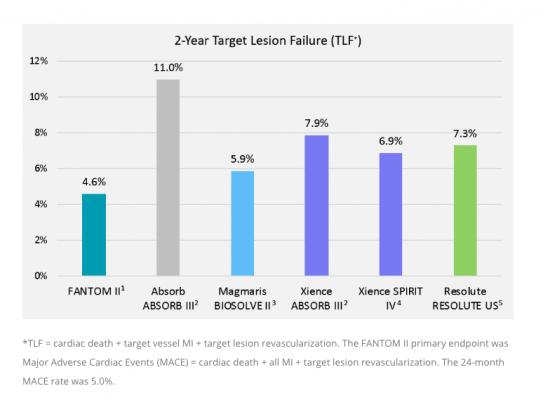
A comparison of target lesion failure (TLF) of the Reva bioresorbable stent to the Absorb and top performing metallic DES.
October 12, 2018 – Reva Medical presented four key data sets demonstrating the capabilities of the company’s Fantom bioresorbable scaffold (BRS) at the 2018 Transcatheter Cardiovascular Therapeutics (TCT) conference. The presentations included new procedural data from an indication expansion study in patients experiencing acute heart attacks as well as positive clinical and imaging results of the Fantom BRS through two years.
FANTOM STEMI Procedural Results
New data from the FANTOM STEMI pilot study showed procedural success and clinical utility of the Fantom bioresorbable scaffold in a series of nine patients with acute heart attacks called ST-segment elevated myocardial infarction (STEMI). Patients experiencing a STEMI are a new patient population for Fantom. While these patients have a higher risk of complications than stable patients, the characteristics of their arterial blockages are typically well suited to BRS. The data were presented by Dr. Lukasz Koltowski from the Medical University of Warsaw in Warsaw, Poland.
“The first priority when treating heart attack patients is removing the arterial blockage to restore blood flow to the heart,” said Koltowski. “The data presented today demonstrate that Fantom, which is X-ray visible and easy to use, works effectively during these emergency procedures. Many heart attack patients are young with single blockages in their arteries, and the Fantom bioresorbable scaffold creates an opportunity for recovery without the risk of a permanent metal drug-eluting stent.”
FANTOM II Clinical Results
Two-year clinical results from the FANTOM II study were presented by Yuichi Saito, M.D., from the Yale University School of Medicine in New Haven, Conn. The data demonstrated safety and efficacy of Fantom at two years with the following outcomes:
• Low 5 percent rate of major adverse cardiac events (MACE)
• A single very late scaffold thrombosis event for a rate of 0.4 percent
FANTOM II Imaging Results
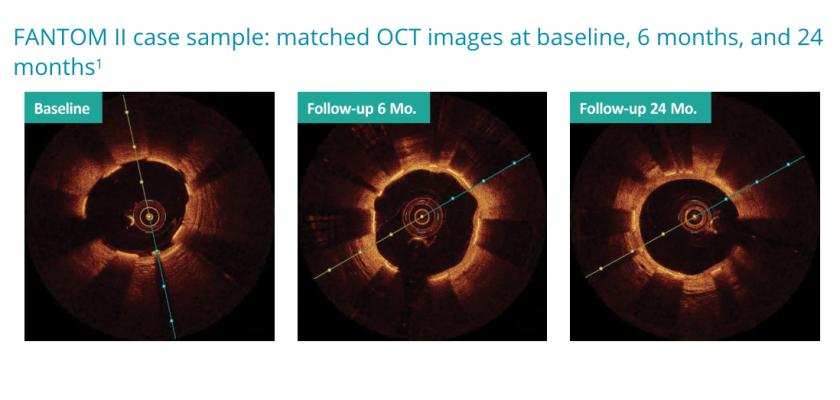
Two-year optical coherence tomography (OCT) imaging results from the FANTOM II study were presented by Dr. Neils Holm from the Aarhus University Hospital in Aarhus, Denmark. The data showed an excellent healing profile for Fantom with sustained vessel lumen patency and no evidence of chronic scaffold recoil through two years.
FANTOM Clinical Review
Dr. Ulf Landmesser, professor of cardiology at Charité Universitätsmedizin Berlin, Germany delivered a comprehensive presentation of the Fantom BRS program. In addition to reviewing available clinical data, Landmesser provided an update on the Fantom Post Market Trial which is currently enrolling in Europe to evaluate
the safety of Fantom in routine clinical practice. The presentation materials delivered at the conference are available in the investor relations section of Reva’s website at www.ir.revamedical.com.
About Fantom and Fantom Encore
Fantom and Fantom Encore are sirolimus-eluting bioresorbable scaffolds developed as alternatives to metallic stents for the treatment of coronary artery disease. Scaffolds provide restoration of blood flow, support the artery through the healing process and then disappear (or resorb) from the body over a period of time. This resorption is intended to allow the return of natural function of the artery and reduce the risk of adverse events associated with a permanent metallic drug-eluting stent. Fantom and Fantom Encore are the only coronary bioresorbable scaffolds made from Tyrocore, Reva’s proprietary tyrosine-derived polymer designed specifically for vascular scaffold applications. Tyrocore is inherently radiopaque, making Fantom and Fantom Encore visible under X-ray fluoroscopy. Fantom and Fantom Encore are designed with thin struts while maintaining strength and with distinct ease-of-use features such as X-ray visibility and expansion with one continuous inflation.
For more information: www.revamedical.com


 January 05, 2026
January 05, 2026 









