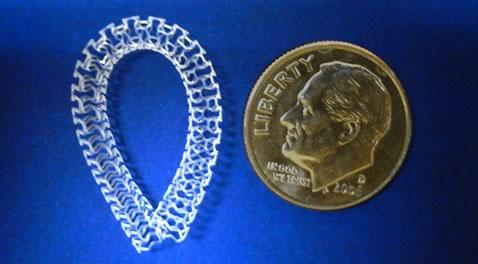
October 25, 2017 — Amaranth Medical will provide an update at the annual Transcatheter Cardiovascular Therapeutics (TCT) meeting, Oct. 29-Nov. 2 in Denver, on the company’s full line of sirolimus-eluting bioresorbable scaffold (BRS) products during a satellite breakfast meeting and in an oral presentation on Oct. 31.
New data to be presented at the meeting include nine-month imaging results from 12 of the 70 patients enrolled in the RENASCENT-III study of Magnitude, the world’s first clinically tested sub-100-micron BRS. Amaranth recently completed enrollment in RENASCENT-III and has commenced plans to submit an application for CE Mark in early 2018.
Amaranth’s TCT presentations will also include previously reported nine-month follow-up results from the 60‑patient RENASCENT-II study of Aptitude, a BRS with a strut thickness of 115 microns. Amaranth submitted an application for CE Mark for Aptitude and is prepared to launch the product in the European market in 2018 pending CE Mark approval.
Juan F. Granada, M.D., co-principal investigator of the study, commented, “These presentations will provide an excellent review of the capabilities of Amaranth’s BRS technology, from Fortitude, the company’s first-generation BRS, to Magnitude, the thinnest BRS ever tested in clinical trials to date. With its 98-micron strut thickness, Magnitude could represent a significant advance in the space.”
Two-year follow-up safety and efficacy results from the 150‑micron Fortitude sirolimus-eluting BRS will also be presented for the first time. These results will provide insight into the long-term safety and biocompatibility of the Amaranth pipeline of products.
The company will also be providing a development update on an 80-micron BRS, which is expected to enter clinical studies in 2018. A scaffold of this strut thickness could potentially rival the characteristics of 80-to-100‑micron drug-eluting metal stents (DES), offering patients a product with all the advantages of a stent without the risks associated with a permanent implant.
Antonio Colombo, M.D., director of the Hemodynamics Division at Ospedale San Raffaele in Milan, Italy and co-principal investigator, added, “I have felt for some time that the next critical improvement in BRS performance will come from a decrease in strut thickness. The 98-micron Magnitude and its 80-micron successor continue to challenge our perceptions of what is possible with bioresorbable technology.”
For more information: www.amaranthmedical.com


 November 14, 2025
November 14, 2025 









