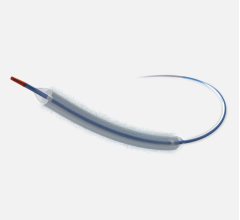
January 2, 2023 — The U.S. Food and Drug Administration (FDA) has approved the BioFreedom Drug Coated Coronary Stent (DCS) System – P190020 from Biosensors International. The BioFreedom Drug Coated Coronary Stent System is intended to treat a narrowed blood vessel (coronary artery) caused by coronary artery disease. The system consists of a catheter delivery system and a 316L stainless steel metal stent. The stent is coated with the drug Biolimus A9. This stent does not contain any additional elements called polymers or carriers to control the release of the drug.
A doctor inserts the BioFreedom Drug Coated Coronary Stent System’s delivery balloon catheter into a blood vessel in a patient’s arm or groin. The stent is then positioned at the site of the coronary artery and the balloon is inflated. The stent expands and is pressed against the coronary artery wall. The stent remains permanently implanted within the coronary artery to help keep it open and improve the supply of blood and oxygen to the heart. The drug (Biolimus A9) is released over time from the BioFreedom stent surface into the coronary artery wall to help prevent re-narrowing, which sometimes occurs after the stent is implanted.
The BioFreedom Drug Coated Coronary Stent System is used in people who have narrowed coronary arteries and are at high risk for bleeding due to previous conditions including:
- Advanced age
- The need for chronic or lifelong anticoagulants (blood thinning medicines such as heparin and others that slow down the process of making blood clots)
- History of major bleeding, stroke, or kidney (renal) insufficiency.
In the primary clinical study, 1,203 patients at high risk for bleeding were treated using the BioFreedom stent and were able to stop taking one of their two antiplatelet therapy medicines (blood thinning medicines such as aspirin and others that prevent blood cells called platelets from clumping together to make a clot) after one month. The results showed that people with the stent who stopped taking one blood thinner one month after stent implant, but kept taking aspirin, were not at increased risk of death or heart attack (myocardial infarction) in the first year, compared to people treated with a non-drug coated stent in an earlier study.
The BioFreedom DCS System should not be used:
- If the patient cannot take blood-thinning medications (also called antiplatelet or anticoagulant therapy)
- If a doctor decides that the coronary artery blockage will not allow complete inflation of the angioplasty balloon.
- If there is a known hypersensitivity (allergy) or other reason not to use Biolimus A9 or the structurally related compounds such as stainless steel, nickel or other metal ions found in 316L.
- If there is a known hypersensitivity (allergy) or other reason not to use contrast agents that will be used for the implantation of the stent.
For more information: www.fda.gov
Related Coronary Stent Content:
The Next Step in Bioresorbable Stent Technologies
VIDEO: The Current State of Bioresorbable Stents in 2018 — Interview with Patrick Serruys, M.D.
VIDEO: Use of the Tryton Dedicated Bifurcation Stent — Interview with Philippe Genereux, M.D.
VIDEO: What Went Wrong With the Absorb Stent? — Interview with Ajay Kirtane, M.D.

 August 22, 2025
August 22, 2025