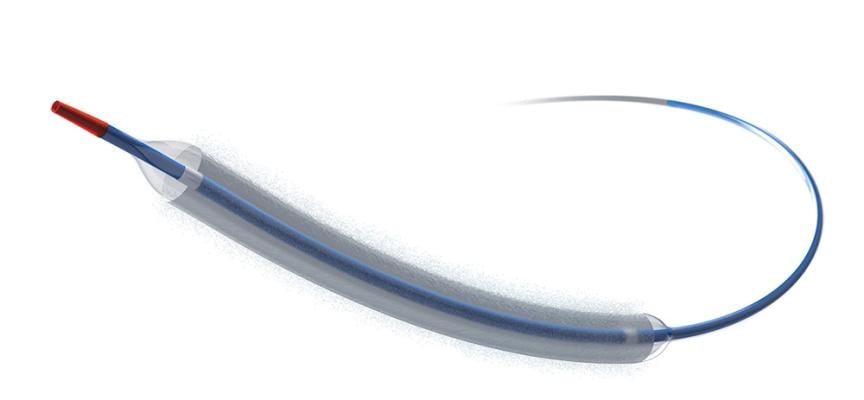
March 1, 2024 — Boston Scientific Corporation today announced it has received U.S. Food and Drug Administration (FDA) approval for the AGENT Drug-Coated Balloon (DCB), which is indicated to treat coronary in-stent restenosis (ISR) in patients with coronary artery disease. ISR is the obstruction or narrowing of a stented vessel by plaque or scar tissue.
"With more than 100,000 patients treated globally to date in both clinical and commercial settings, we are very pleased to introduce this proven therapy as the first drug-coated coronary balloon in the U.S," said Lance Bates, president, Interventional Cardiology Therapies, Boston Scientific. "The AGENT DCB addresses a critical unmet need by providing a dedicated treatment option for the challenging condition of ISR and we look forward to offering U.S. physicians the opportunity to treat their patients with this novel device."
While the stenting of coronary lesions continues to show a substantial improvement in quality of life for patients with coronary artery disease, ISR still encompasses 10 percent of percutaneous coronary interventions in the U.S.1,2 Serving as an alternative to traditional therapies such as balloon angioplasty, additional layers of stenting or radiation, the AGENT DCB is a paclitaxel-coated balloon catheter that transfers a therapeutic dose of drug to the vessel wall to help prevent ISR reoccurrence.
Following Breakthrough Device Designation granted for the technology by the FDA in 2021, the approval was supported by positive results from the multicenter, prospective, randomized controlled AGENT IDE trial, which enrolled 600 patients at 40 U.S. sites.3 In the prespecified interim analysis of the first 480 patients enrolled, the study met the primary endpoint of target lesion failure (TLF) at 12 months with the AGENT DCB demonstrating statistical superiority to uncoated balloon angioplasty (17.9% vs. 28.7%; P=0.006).4,5 Findings also included zero definite/probable cases of clotting within the stent (0.0% vs. 3.9%, P=0.001), a 49% risk reduction in heart attack at the target vessel (6.4% vs. 12.3%, P=0.03) and low adverse event rates at 12 months.
"The AGENT IDE trial demonstrated that the AGENT DCB is an effective and safe treatment option for coronary in-stent restenosis, even in a high-risk population, which included many individuals with multi-layer stents or diabetes," said principal investigator Dr. Robert W. Yeh, section chief of interventional cardiology at the Beth Israel Deaconess Medical Center. "Treating ISR has been challenging in the U.S. with limited therapies available, and this new technology will help physicians reduce the risk of restenosis without radiation or introducing additional metal layers, which do not provide an adequate result for some patients."
The AGENT DCB is available in Europe, parts of Asia Pacific and Latin America for the treatment of patients with ISR and previously untreated small vessel coronary disease. Boston Scientific plans to launch the technology in the U.S. in the coming months.
For more information: www.bostonscientific.com


 January 15, 2026
January 15, 2026 









