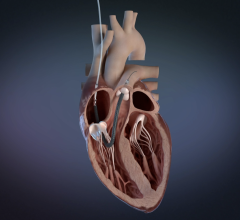
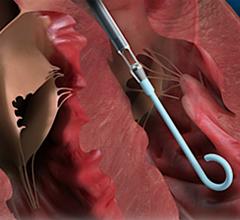
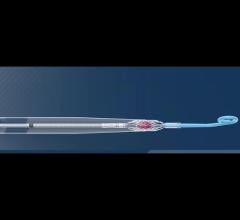
Xeltis synthetic restorative pulmonary heart valve evolves into a fully functioning, natural heart valve through colonization by the patient’s own tissue. (Photo: Business Wire)
June 13, 2024 — Xeltis, a leading developer of transformative implants that enable the natural creation of living and long-lasting vessels, announces that it has gained approval from the US Food and Drug Administration (FDA) for an Investigational Device Exemption (IDE) submission to begin enrolling patients into a pivotal study for aXess.
aXess is a restorative conduit that enables the creation of a new, permanent, living vessel for hemodialysis vascular access, combining the safety and patency of an arteriovenous fistula (AVF), with the speed to treatment of an arteriovenous graft (AVG). The aXess vascular access conduit offers an improved dialysis patient experience and avoids the frequent reinterventions and complications, such as infections, faced by many renal disease patients.
Xeltis’ groundbreaking implants platform is an important development in vascular replacement technology as, over time, its implants are gradually replaced by the patients’ own living healthy tissue. Xeltis’ novel technology has already treated over 100 patients across different clinical trials.
Eliane Schutte, Chief Executive Officer, Xeltis commented: “We have already shown outstanding 12-month data from our first-in-human study in Europe and are looking forward to starting this pivotal trial in the US. We are very proud of the potential for aXess to transform the field of vascular access by stopping the cycle of interventions and infections and bringing our unique restorative solution to hemodialysis patients worldwide.”
Paulo Neves, Chief Medical Officer, Xeltis said: “Our focus is on improving the outcomes for patients on dialysis. aXess offers this potential through its avoidance of the reinterventions and complications associated with other vascular access solutions. This pivotal study is important in assessing and demonstrating this and marks a significant milestone in our clinical strategy in the US.”
The US-based pivotal trial follows strong 12-month data from the first-in-human trial in Europe in comparison to hemodialysis vascular access solutions. aXess is also undergoing an EU pivotal trial, recruiting up to 110 patients in nine EU countries.
For more information: https://xeltis.com/


 October 23, 2023
October 23, 2023