October 16, 2020 — The Restore EF Study demonstrates the use of contemporary best practices, including attempting a more complete revascularization with Impella-supported high-risk percutaneous coronary intervention (PCI), is associated with significant improvement of left ventricular ejection fraction (LVEF), heart failure symptoms, and anginal symptoms at follow up. The interim analysis was presented today by Mitul Patel, M.D., an interventional cardiologist at UC San Diego Health, at the 2020 Transcatheter Cardiovascular Therapeutics (TCT) Connect virtual symposium of the Cardiovascular Research Foundation (CRF).
The ongoing, multi-center, prospective, single-arm study enrolled 193 consecutive qualified patients who underwent a Protected PCI procedure with Impella between September 2019 and September 2020 at 19 hospitals in the United States, representing a variety of hospital settings including rural, urban, community and academic centers. The interim analysis showed:
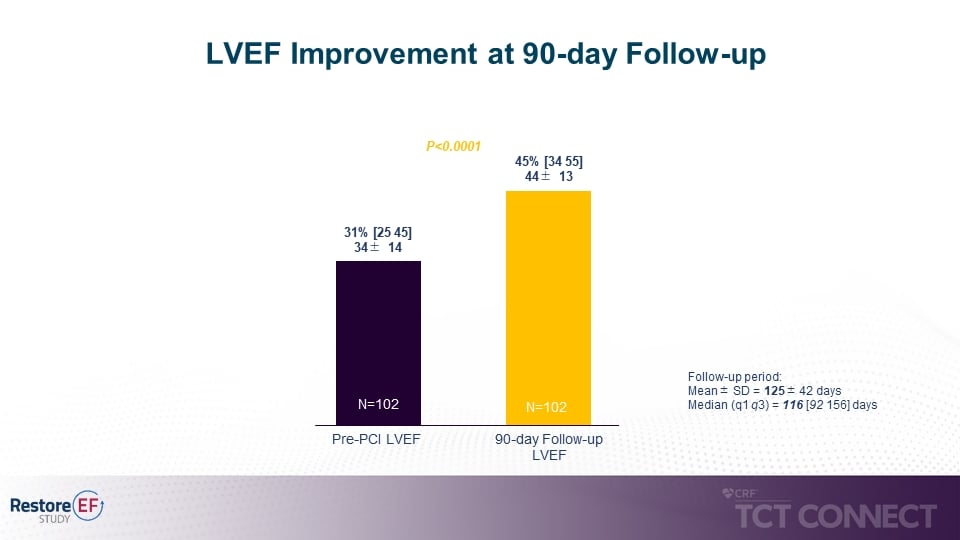
• Significant median LVEF improvement from baseline to 90-day follow up (31% to 45% p<0.0001). LVEF improvement at 90 days is the study’s primary endpoint. (see figure 1)
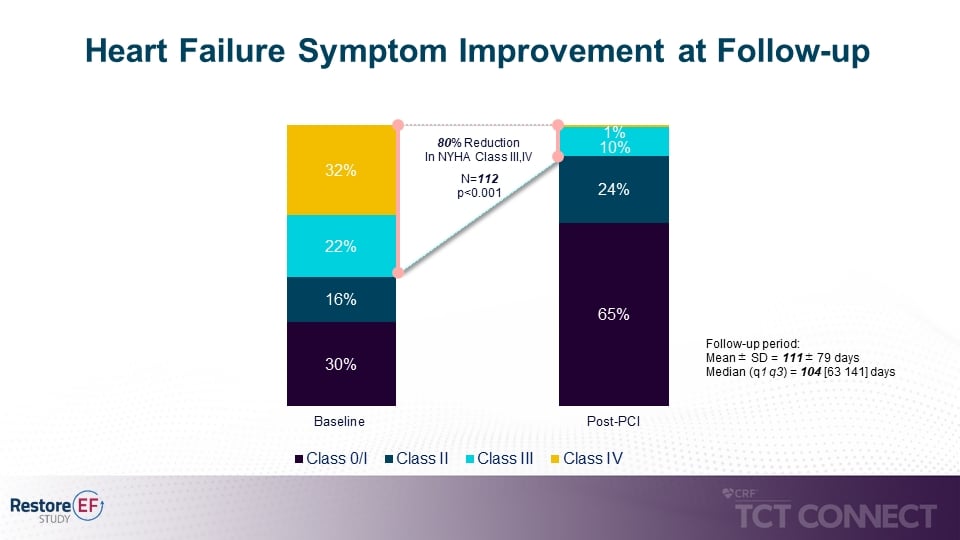
• Significant reduction of heart failure symptoms with 80% reduction in New York Heart Association (NYHA) classification III/IV at follow up (54% to 11% p<0.001). (see figure 2)
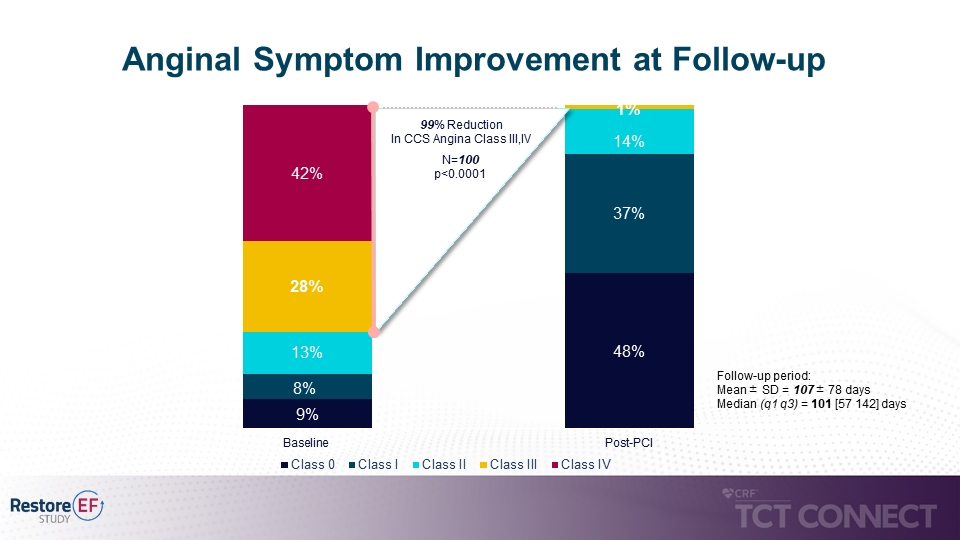
• Significant reduction of anginal symptoms with 99% reduction in Canadian Cardiovascular Society (CCS) classification III/IV at follow up (70% to 1% p<0.0001). (see figure 3)
“Restore EF demonstrates Impella-supported PCI patients have shown a significant LVEF improvement at 90 days. The study also found a significant improvement in heart failure and anginal symptoms assessed with NYHA and CCS functional classifications,” said Dr. Patel. “Taken together, this data validates best practices for treating high-risk PCI patients, including the use of Impella to achieve a complete revascularization in a single setting.”
“High-risk PCI patients often pose a revascularization challenge due to patient comorbidities, poor LV function, and adverse hemodynamics, which drive worse outcomes. This research demonstrates the rationale for using Impella support during high-risk PCI to maintain coronary perfusion and support hemodynamics during periods of myocardial ischemia during long or repeated balloon inflations or atherectomy runs. This allows providers to achieve complete functional revascularization and the best possible outcomes for our patients,” said Jason Wollmuth, M.D., an interventional cardiologist at Providence Health and Vascular Institute and a co-principal investigator of the Restore EF Study.
Restore EF is part of a growing body of evidence demonstrating Protected PCI with Impella is associated with improvements in LVEF and heart failure symptoms. That research includes:
• Burzotta et al., which found Protected PCI with Impella is associated with LVEF improvement in complex high-risk patients at 90 days (27% vs. 33%, p<0.001). The authors also found more complete revascularization is associated with increased LVEF and survival.
• PROTECT II Randomized Controlled Trial, which found Protected PCI with Impella led to a 58% improvement in NYHA class III and IV heart failure symptoms at 90 days (p<0.001). The trial also found, during follow up after Protected PCI with Impella, patients had a 22% improvement in LVEF (p<0.001).
• Maini et al., which found a 17% improvement in LVEF at follow up after a Protected PCI with Impella (p<0.0001).
Findings from Restore EF will be used to inform the study protocol for the upcoming PROTECT IV Randomized Controlled Trial. PROTECT IV will be a prospective, two-arm trial that will compare complete revascularization PCI with Impella to complete revascularization PCI without any planned hemodynamic support. PROTECT IV is part of the Impella clinical evidence pathway to a Class I clinical guideline recommendation for high-risk PCI.
The Restore EF study is sponsored by Abiomed and reflects the company’s commitment to investing in clinical research to improve patient outcomes.
To share best practices in high-risk PCI, Abiomed is hosting a symposium at TCT Connect on Saturday, Oct. 17, at 2 p.m. EDT, titled Protected PCI in COVID-19 Era: The Rise in Importance of Complete Revascularization. The symposium is chaired by Cindy Grines, M.D., chief scientific officer of Northside Hospital Cardiovascular Institute in Atlanta and president of the Society for Cardiovascular Angiography and Interventions (SCAI). It will feature best practices for using percutaneous mechanical circulatory support to enable complete revascularization in high-risk patients.
For additional information: www.abiomed.com
Find additional TCT 2020 news, video and late-breaking studies


 January 05, 2026
January 05, 2026 









