March 1, 2024 — Boston Scientific Corporation today announced it has received U.S. Food and Drug Administration (FDA) approval for the AGENT Drug-Coated Balloon (DCB), which is indicated to treat ...
Drug-Eluting Balloons
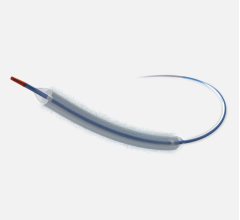
This channel includes news and new technology innovations for drug-coated balloons (DCB), also referred to as drug-eluting balloons. These are used to treat peripheral and coronary artery lesions and restenosis. The balloons carry an antiproliferative drug that is delivered to the wall of arteries when the balloon is expanded. The drug helps prevent neointimal hyperplasia (scar tissue growth) caused by trauma when the vessel segment is treated for atherosclerotic lesions with balloon angioplasty. DCBs can be used to treat hyperplasia in arteriovenous (AV) access fistulae in dialysis patients, where the vessel undergoes repeated trauma from regular punctures. DCBs also are used to treat in-stent restenosis due to scar tissue proliferation inside stents, which can cause a vessel to occlude.
Aug. 21, 2025 — Boston Scientific has initiated the AGENT DCB STANCE trial to assess the safety and effectiveness of the ...
June 18, 2024 — Elixir Medical has announced the company’s novel bioadaptive implant, DynamX Sirolimus-Eluting Coronary ...
April 2, 2024 — Medical device technology developer Concept Medical has announced it has been granted Investigational ...
March 20, 2024 — Biotronik has been granted Breakthrough Device Designation (BDD) from the US Food and Drug ...
March 13, 2024 — In the largest randomized clinical trial and first of its kind to date in the United States, a team led ...
March 1, 2024 — Boston Scientific Corporation today announced it has received U.S. Food and Drug Administration (FDA) ...
November 1, 2023 — The VIVA Foundation has announced the results for the first Late-Breaking Clinical Trial sessions at ...
October 30, 2023 — Boston Scientific Corporation on Friday announced positive 12-month results from the pivotal AGENT ...
September 14, 2023 — BIOTRONIK announced the two-year-results from the BIOLUX P-III BENELUX all-comers registry ...
September 11, 2023 — The first patient has been enrolled in a UK study of large vessel de novo coronary artery disease t ...
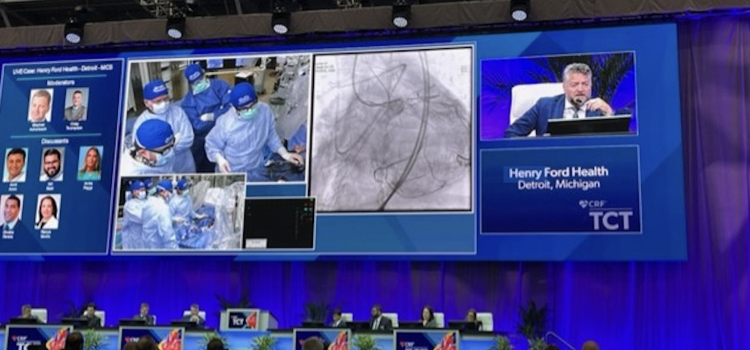
September 6, 2023 — The Cardiovascular Research Foundation (CRF) has announced the TCT 2023 late-breaking clinical ...
August 28, 2023 — Prasugrel monotherapy after percutaneous coronary intervention (PCI) with drug-eluting stents is not ...
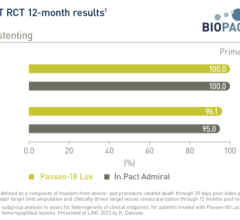
July 14, 2023 — BIOTRONIK announced the one-year subgroup results from the investigator-initiated BIOPACT randomized ...
May 30, 2023 — Twelve-month results from the SELUTION SFA trial have been presented for the first time at the Japan ...
May 30, 2023 — The US FDA, on the 24th of May 2023, granted an Investigational Device Exemption (IDE) approval for Conce ...



 August 22, 2025
August 22, 2025