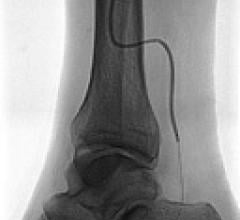
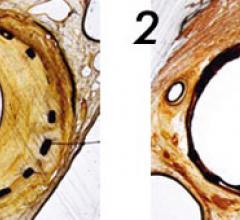
May 17, 2011 - Medrad Inc., a business of Bayer HealthCare, today announced it has received CE Mark for its Cotavance ...
Drug-Eluting Balloons
This channel includes news and new technology innovations for drug-coated balloons (DCB), also referred to as drug-eluting balloons. These are used to treat peripheral and coronary artery lesions and restenosis. The balloons carry an antiproliferative drug that is delivered to the wall of arteries when the balloon is expanded. The drug helps prevent neointimal hyperplasia (scar tissue growth) caused by trauma when the vessel segment is treated for atherosclerotic lesions with balloon angioplasty. DCBs can be used to treat hyperplasia in arteriovenous (AV) access fistulae in dialysis patients, where the vessel undergoes repeated trauma from regular punctures. DCBs also are used to treat in-stent restenosis due to scar tissue proliferation inside stents, which can cause a vessel to occlude.
November 17, 2010 – A drug-eluting balloon (DEB) and a coronary stent have received CE mark approval in Europe ...
September 29, 2010 – Results from a trial studying the effects of a drug-eluting balloon (DEB) on peripheral ...
May 25, 2010 – The first patient has been implanted with a bare metal coronary stent mounted on a drug-eluting ...

Several paclitaxel drug-eluting balloons (DEBs) are currently available on the European market, and several others are ...
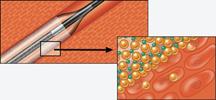
Drug-eluting balloons (DEBs) may offer new options in treating peripheral vessels and restenosis. DEBs already ...

In recent years, many medical device companies saw the underserved lower extremity as an opportunity to enter the ...
February 22, 2010 – As part of a comarketing and trademark license agreement between MEDRAD Inc. and B. Braun ...
January 25, 2010 – Expanding its peripheral vascular disease product offerings, Medtronic Inc. today signed an ...

The focus on balloon angioplasty largely slipped from the interventional limelight a decade ago after the ...
May 19, 2009 - Invatec announced today the availability of its newly CE-marked coronary balloon, the IN.PACT ...
March 16, 2009 – Invatec today announced CE-certification of a new coronary balloon, the IN.PACT Falcon paclitaxel ...
January 13, 2009 - Micell Technologies, a development-stage biomedical device company dedicated to developing ...
October 24, 2007 — Drug-eluting balloons could offer a viable alternative to drug-eluting stents (DES) in the ...

 May 18, 2011
May 18, 2011