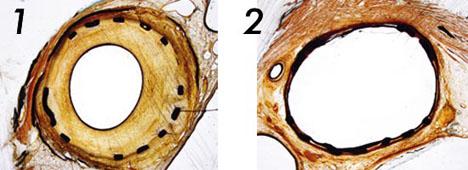
The first photo shows a control in a porcine artery treated with a conventional uncoated balloon, showing massive neointimal proliferation. The second photo shows a different section of the same artery treated with a IN.PACT paclitaxel-coated balloon.
May 19, 2009 - Invatec announced today the availability of its newly CE-marked coronary balloon, the IN.PACT Falcon paclitaxel-eluting PTCA balloon catheter at the EuroPCR Congress 2009 in Barcelona, Spain.
This is one of the first drug-eluting balloons released in Europe designed specifically to treat atherosclerosis in the coronary arteries and underscores Invatec’s commitment to robust scientific research into the reduction of re-intervention rates in the treatment of coronary artery disease (CAD). The IN.PACT Falcon is not FDA-cleared.
“Combining world-class PTCA balloon catheter technology with local drug administration is a fascinating new concept for the treatment of certain coronary lesions such as in-stent restenosis (ISR), small vessel disease (SVD), bifurcations and potentially other lesions where conventional balloons, stents and even drug eluting stents may not be ideal,” said Professor Eberhard Grube, chief, department of cardiology/angiology at The Heart Center, Siegburg, Germany. “A drug eluting balloon such as the IN.PACT Falcon that elutes a known and effective drug such as paclitaxel holds much promise as an effective treatment option for patients.”
IN.PACT Falcon combines the currently marketed, performance-leading Falcon line of PTCA balloon catheters with Invatec’s proprietary IN.PACT technology platform and FreePac coating. FreePac is a proprietary, natural coating that frees and separates paclitaxel molecules and facilitates their absorption into the wall of the artery. The FreePac coating was developed in close collaboration with the researchers who pioneered drug-eluting balloon therapy, Ulrich Speck, Ph.D., department of radiology at Charite Mitte, Berlin and Bruno Scheller, M.D., University Hospital, department of internal medicine, Homburg/Saar.
For more information: visit www.invatec.com


 June 13, 2024
June 13, 2024 









