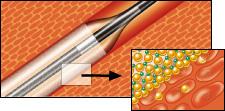
March 16, 2009 – Invatec today announced CE-certification of a new coronary balloon, the IN.PACT Falcon paclitaxel-eluting PTCA balloon catheter, which is one of the first drug-eluting balloons designed specifically to treat atherosclerosis in the coronary arteries.
IN.PACT Falcon features FreePac, a proprietary, natural coating that frees and separates paclitaxel molecules and facilitates their absorption into the wall of the artery. The FreePac coating was developed in close collaboration with the researchers who pioneered drug-eluting balloon therapy, Ulrich Speck, Ph.D., department of radiology at Charite Mitte, Berlin, and Bruno Scheller, M.D., University Hospital Department of Internal Medicine, Homburg/Saar.
“The drug-eluting balloon concept has already shown its potential to reduce re-intervention rates for patients with coronary atherosclerosis in clinical trials in a selected patient population,” said Prof. Scheller and Prof. Speck “Therefore the launch of efficacious matrix coatings on modern coronary balloon catheters by renowned medical device manufacturers is a major milestone in the development of appropriate ways to treat coronary atherosclerosis. It is also an essential precondition to incorporate clinical practice as the driving force for further improvements.”
“Combining world-class PTCA balloon catheter technology with local drug administration is a fascinating new concept for the treatment of certain coronary lesions such as in-stent restenosis (ISR), bifurcations, small vessel disease (SVD) and potentially other lesions where conventional balloons, stents and even drug eluting stents may not be ideal. A drug eluting balloon such as the IN.PACT Falcon that elutes a known and effective drug such as paclitaxel holds much promise as an effective treatment option for patients.”
In addition to IN.PACT Falcon paclitaxel-eluting balloon, Invatec recently launched IN.PACT Amphirion, its first drug-eluting balloon designed specifically for the treatment of atherosclerosis in arteries located below the knee. The launch of IN.PACT Falcon is the company’s second drug eluting balloon platform.
For more information: www.invatec.com


 June 13, 2024
June 13, 2024 









