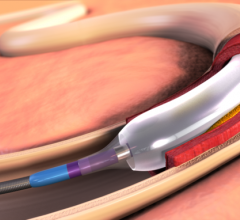
December 19, 2014 — Cardionovum GmbH announced that it has initiated a 150-patient clinical study of its novel paclitaxe ...
Drug-Eluting Balloons
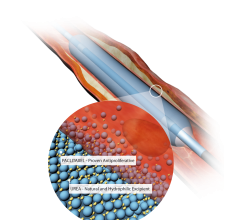
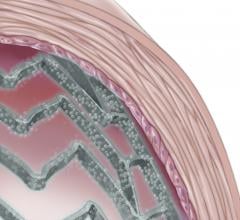
This channel includes news and new technology innovations for drug-coated balloons (DCB), also referred to as drug-eluting balloons. These are used to treat peripheral and coronary artery lesions and restenosis. The balloons carry an antiproliferative drug that is delivered to the wall of arteries when the balloon is expanded. The drug helps prevent neointimal hyperplasia (scar tissue growth) caused by trauma when the vessel segment is treated for atherosclerotic lesions with balloon angioplasty. DCBs can be used to treat hyperplasia in arteriovenous (AV) access fistulae in dialysis patients, where the vessel undergoes repeated trauma from regular punctures. DCBs also are used to treat in-stent restenosis due to scar tissue proliferation inside stents, which can cause a vessel to occlude.
Nov. 14, 2014 — Covidien releases 12-month results of the DEFINITIVE AR study, the first randomized study designed to ...
Nov. 13, 2014 — Optimizing the treatment of coronary artery disease with a new foundation for future stent innovations ...
Nov. 10, 2014 — For the treatment of peripheral artery disease in leg arteries above the knee, the IN.PACT Admiral drug ...
October 31, 2014 — Metro Health (Michigan) cardiovascular specialist Jihad Mustapha, M.D., is one of the first ...
October 29, 2014 — Restenosis, the recurrence of narrowing of the arteries after stenting, is a common risk of this ...
October 14, 2014 — Presented for the first time at the 2014 Transcatheter Cardiovascular Therapeutics (TCT) conference ...
October 13, 2014 — C. R. Bard Inc. announced the U.S. Food and Drug Administration (FDA) approval of the Lutonix 035 ...

July 28, 2014 — Boston Scientific Corp. received CE mark for the Ranger Paclitaxel-coated PTA Balloon Catheter. The ...

June 16, 2014 — C. R. Bard Inc. announced that the U.S. Food and Drug Administration’s (FDA) Circulatory System Devices ...
May 23, 2014 — The first drug-eluting balloon to go up for review by the U.S. Food and Drug Administration (FDA) will be ...
April 16, 2014 — Patients with peripheral artery disease in the upper leg experienced significantly better outcomes at ...
March 6, 2014 — Despite good immediate results, in up to 40 percent of patients, obstructed arteries in the leg treated ...


 December 19, 2014
December 19, 2014