May 23, 2014 — The first drug-eluting balloon to go up for review by the U.S. Food and Drug Administration (FDA) will be discussed at the next Circulatory System Devices Panel of the Medical Devices Advisory Committee on June 12 in Germantown, Md.
The committee will discuss, make recommendations and vote on information related to the premarket approval application for the Lutonix 035 drug-coated balloon (DCB). The device was developed by Lutonix Inc., which C.R. Bard acquired in December 2011. The Lutonix drug-coated balloon received European CE mark approval in 2011 and has been the subject of safety and effectiveness testing in the LEVANT 2 U.S. pivotal trial.
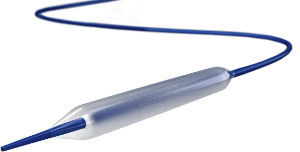
The balloon is an over-the-wire percutaneous transluminal angioplasty (PTA) catheter with a paclitaxel-based drug coating on the surface of the balloon. The Lutonix DCB is compatible with a 0.035-inch guidewire and has balloon sizes ranging from 4 to 6 mm in diameter and 40 to 100 mm in length. The catheter is available in 75, 100 and 130 cm working lengths.
The proposed indications for use are for improving luminal diameter for the treatment of obstructive de novo or non-stented restenotic lesions (?15cm in length) in native femoropopliteal arteries having reference vessel diameters of 4 mm to 6 mm.
LEVANT 2 randomized 476 patients presenting with claudication or ischemic rest pain and an angiographically significant lesion in the superficial femoral or popliteal artery and a patent outlflow artery to the foot. After a successful protocol-defined pre-dilation, subjects unlikely to require a stent based on strict angiographic criteria were randomized two-to-one to the treatment with either a drug-coated balloon (DCB) or PTA alone with a standard balloon.
The six-month data was presented at TCT (Transcatheter Cardiovascular Therapeutics) 2013. At six months by Kaplan-Meier time-to-event analysis, primary patency of the treated vessel was higher among patients treated with a DCB (92.3 percent versus 82.7 percent). Patients treated with DCB experienced similar freedom from major adverse events compared to the PTA group (94 percent in the DCB group and 94.1 percent in the PTA group). Repeat revascularization rates at this interim time point were low and consistent in both groups.
“During angioplasty, DCBs are designed to deliver an anti-proliferative drug directly to the tissues of the treated vessel wall, thus inhibiting neointimal hyperplasia and restenosis without the need for a permanent foreign body implant,” said Kenneth Rosenfield, M.D., section head, vascular medicine and intervention, chairman, STEMI and Acute MI Quality Improvement Committee, Massachusetts General Hospital and co-primary investigator of the study. “These findings are an important step toward making this novel treatment available to patients in the United States.”
For more information: www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/default.htm


 January 05, 2026
January 05, 2026 









