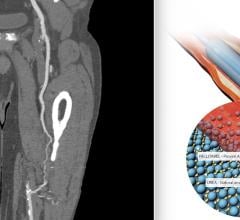
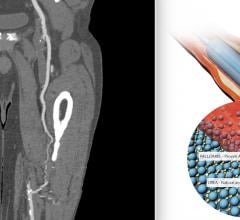
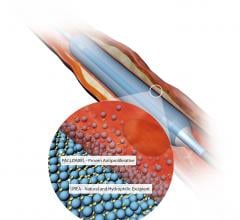
June 8, 2022 — The first patient has been enrolled in the FDA IDE BTK (Below-the-Knee) SELUTION4BTK clinical trial ...
Drug-Eluting Balloons
This channel includes news and new technology innovations for drug-coated balloons (DCB), also referred to as drug-eluting balloons. These are used to treat peripheral and coronary artery lesions and restenosis. The balloons carry an antiproliferative drug that is delivered to the wall of arteries when the balloon is expanded. The drug helps prevent neointimal hyperplasia (scar tissue growth) caused by trauma when the vessel segment is treated for atherosclerotic lesions with balloon angioplasty. DCBs can be used to treat hyperplasia in arteriovenous (AV) access fistulae in dialysis patients, where the vessel undergoes repeated trauma from regular punctures. DCBs also are used to treat in-stent restenosis due to scar tissue proliferation inside stents, which can cause a vessel to occlude.
May 27, 2022 — Medtronic today announced approval from the U.S. Food and Drug Administration (FDA) for the IN.PACT 018 ...
May 27, 2022 — The SELUTION SLR (Sustained Limus Release) is a novel sirolimus-eluting balloon that provides a ...
May 20, 2022 — New long-term data from the Safety Assessment of Femoropopliteal Endovascular Treatment With PAclitaxel ...
May 19, 2022 — One year outcomes from the Disrupt PAD III Trial comparing intravascular lithotripsy (IVL) with a drug ...
March 31, 2022 — In accordance with its commitment to patient safety, Medtronic recently voluntarily recalled a subset ...
November 10, 2021 — Biosensors International Group Ltd., a developer and manufacturer of innovative medical devices ...
October 20, 2021 — 18-month results from the PRESTIGE* Below-the-Knee (BTK) study have been presented as a late-breaking ...
June 7, 2021 — A couple years ago a study showed a mortality safety signal in patients who underwent peripheral artery ...
May 20, 2021 — Boston Scientific Corporation announced it has initiated the AGENT IDE trial for the Agent Drug-Coated ...

The first devices developed for interventional cardiology were percutaneous transluminal coronary angioplasty (PTCA) ...
March 12, 2021 — Philips Healthcare announced the final, five-year results of two major randomized controlled trials ...
November 3, 2020 — The U.S. Food and Drug Administration (FDA) has cleared the Boston Scientific Ranger Drug-coated ...

October 18, 2020 – A large subgroup analysis of a randomized clinical trial showed neither a mortality risk nor benefit ...
October 18, 2020 – The first results from the IN.PACT Below the Knee (BTK) Study, a feasibility study assessing the ...


 June 08, 2022
June 08, 2022