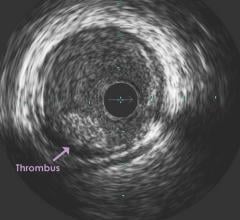
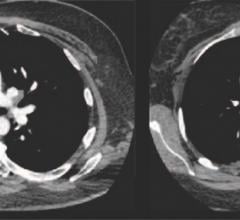
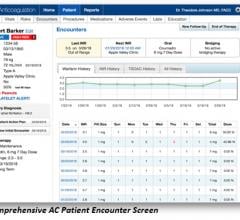
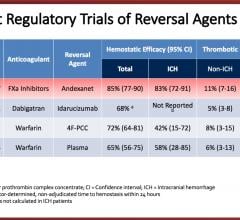
September 7, 2018 — A new Phase 2 clinical trial looks to confirm the efficacy and safety of Thrombolytic Science LLC’s ...
Antiplatelet and Anticoagulation Therapies
This channel includes news and new technology innovations for cardiovascular pharmaceutics. This includes antiplatelet agents, anticoagulation drugs, international normalized ratio (INR) testing, oral anticoagulants, IV administered drugs such as Heprin, and dual antiplatelet therapy (DAPT). The most commonly used anticoagulant is warfarin, which available in generic form at a low cost. However, it has a narrow therapeutic window and its effectiveness is altered by food containing vitamin K. To regulate warfarin, regular INR testing is needed. The newer anticoagulation drugs are referred to as novel oral anticoagulant (NOAC). However, since some of these drugs are now more than six years old, they are commonly referred to as non-vitamin K oral anticoagulant (NOAC), and the newest term, direct oral anticoagulant (DOAC). NOAC or DOAC agents have much larger therapeutic windows, do not require INR testing. Aspirin and clopidogrel (Plavix) are the most commonly used antiplatelet agents for the prevention of heart attacks and stroke. They are often prescribed together as DAPT.
September 5, 2018 — Aspirin prevented serious vascular events in patients with diabetes who did not already have ...
August 31, 2018 — Use of an oral anticoagulant in medically ill patients for 45 days following hospital discharge ...
June 29, 2018 — Results from the MERCURY PE study showed that low-risk pulmonary embolism (PE) patients treated with Xar ...
June 27, 2018 — Results from a 902-person, five-country survey of people living with atrial fibrillation (AF) reinforce ...
June 7, 2018 — Results from an international clinical trial show that combining clopidogrel and aspirin following a ...
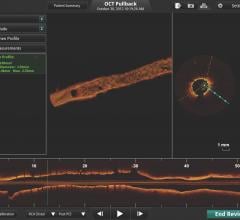
May 29, 2018 – Investigators recently unveiled clinical data from the independently run Onyx 1-Month OCT Study showing ...
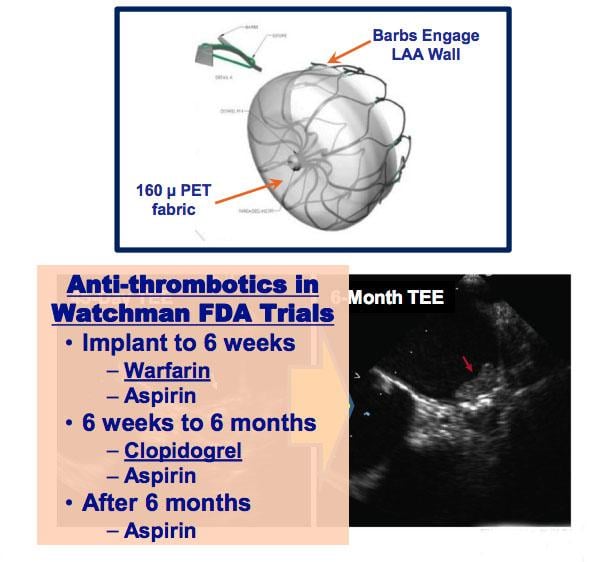
May 18, 2018 — Left atrial appendage closure (LAAC) with the transcatheter Watchman device prevents thromboembolism from ...
May 18, 2018 — Nearly half of patients prescribed warfarin and just under one third of those using newer direct oral ...
May 15, 2018 – Results of the AVIATOR 2 international registry data show a discrepancy between physician perception and ...
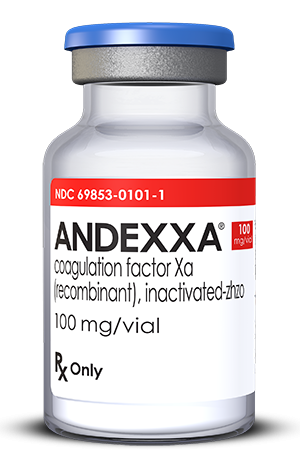
May 7, 2018 — The U.S. Food and Drug Administration (FDA) has approved Portola Pharmaceuticals' Andexxa, the first ...
April 30, 2018 — Point of Care Decision Support (PCDS) announced the release of a new version 2.0 of their ...

There were several notable presentations of new data on cardiovascular technologies at the recent 2018 American College ...
March 22, 2018 — The experimental drug andexanet was associated with control of serious bleeding in patients taking a ...
March 20, 2018 — Treatment with the blood-thinning drug dabigatran significantly reduced the risk of death, heart attack ...

 September 07, 2018
September 07, 2018