November 14, 2016 — Two differing blood clot prevention medications are just as safe and effective for patients ...
Antiplatelet and Anticoagulation Therapies
This channel includes news and new technology innovations for cardiovascular pharmaceutics. This includes antiplatelet agents, anticoagulation drugs, international normalized ratio (INR) testing, oral anticoagulants, IV administered drugs such as Heprin, and dual antiplatelet therapy (DAPT). The most commonly used anticoagulant is warfarin, which available in generic form at a low cost. However, it has a narrow therapeutic window and its effectiveness is altered by food containing vitamin K. To regulate warfarin, regular INR testing is needed. The newer anticoagulation drugs are referred to as novel oral anticoagulant (NOAC). However, since some of these drugs are now more than six years old, they are commonly referred to as non-vitamin K oral anticoagulant (NOAC), and the newest term, direct oral anticoagulant (DOAC). NOAC or DOAC agents have much larger therapeutic windows, do not require INR testing. Aspirin and clopidogrel (Plavix) are the most commonly used antiplatelet agents for the prevention of heart attacks and stroke. They are often prescribed together as DAPT.
November 10, 2016 — Siemens Healthineers announced U.S. Food and Drug Administration (FDA) 510(k) clearance for a hand ...
October 28, 2016 — Medicure Inc. recently received U.S. Food and Drug Administration (FDA) market clearance for its new ...
October 25, 2016 — A recent study from University of Alabama at Birmingham (UAB) researchers published in PLOS ONE compa ...
September 1, 2016 — First outcome results from the GLORIA-AF Registry show that treatment with Pradaxa (dabigatran ...
August 24, 2016 — Whether severe trauma occurs on the battlefield or the highway, saving lives often comes down to stopp ...
August 18, 2016 — Researchers at the Peter Munk Cardiac Centre, Toronto, and at Mayo Clinic are leading the Tailored ...
August 16, 2016 — Warfarin prescribed to prevent strokes in atrial fibrillation may not adequately control blood ...

August 5, 2016 — Here are the top 20 most popular current content on the Diagnostic and Interventional Cardiology ...
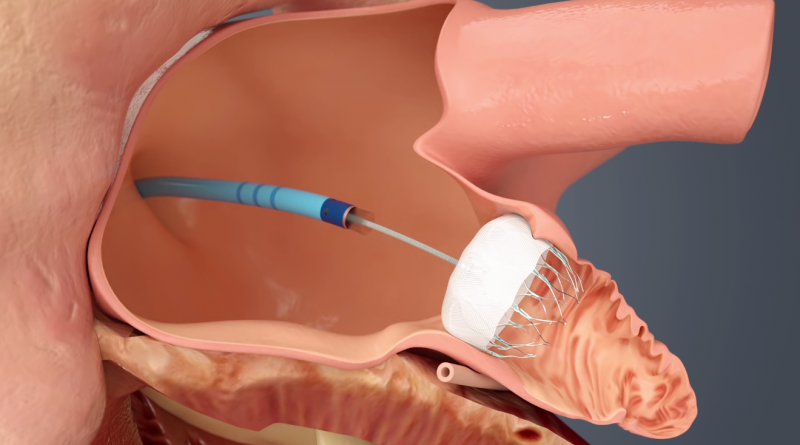
Patients with atrial fibrillation (AF or Afib) are high risk for stroke due to the formation of thrombus emboli in the ...
July 26, 2016 — Hematology researchers at The Children’s Hospital of Philadelphia (CHOP) have developed a novel ...
July 14, 2016 — BBMK Technologies recently announced the launch of ClotMD, a cloud-based application and mobile solution ...
July 12, 2016 — Following a collaborative process with the U.S. Food and Drug Administration (FDA), Alere Inc. will be ...

With the recent introduction of several novel oral anticoagulants (NOACs) on the U.S. market that have been billed by ...
June 24, 2016 — More than 1 in 3 atrial fibrillation (AF) patients at intermediate to high risk for stroke are treated ...

 November 14, 2016
November 14, 2016