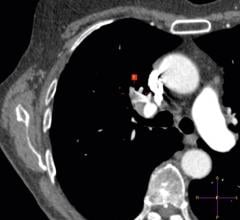
December 17, 2015 — The ROCKET AF Clinical Trial Executive Committee announced its secondary analysis of the phase III ...
Antiplatelet and Anticoagulation Therapies
This channel includes news and new technology innovations for cardiovascular pharmaceutics. This includes antiplatelet agents, anticoagulation drugs, international normalized ratio (INR) testing, oral anticoagulants, IV administered drugs such as Heprin, and dual antiplatelet therapy (DAPT). The most commonly used anticoagulant is warfarin, which available in generic form at a low cost. However, it has a narrow therapeutic window and its effectiveness is altered by food containing vitamin K. To regulate warfarin, regular INR testing is needed. The newer anticoagulation drugs are referred to as novel oral anticoagulant (NOAC). However, since some of these drugs are now more than six years old, they are commonly referred to as non-vitamin K oral anticoagulant (NOAC), and the newest term, direct oral anticoagulant (DOAC). NOAC or DOAC agents have much larger therapeutic windows, do not require INR testing. Aspirin and clopidogrel (Plavix) are the most commonly used antiplatelet agents for the prevention of heart attacks and stroke. They are often prescribed together as DAPT.
November 24, 2015 —The U.S. Court of Appeals has overturned a federal circuit court’s decision in The Medicines Company ...
November 24, 2015 – The U.S. Food and Drug Administration (FDA) approved Boehringer Ingelheim Pharmaceuticals’ ...

One-third of ischemic strokes are classified as cryptogenic. The classic risk factors for stroke are usually absent ...
November 12, 2015 — Preventing blood clots with drugs such as heparin has become a common practice for fighting some ...
November 11, 2015 — Brigham and Women’s Hospital in Boston and Boehringer Ingelheim announced the results of a new ...
November 3, 2015 — Pulmonary embolism (PE) is a potentially life-threatening condition that occurs, for example, when a ...
October 23, 2015 — A randomized trial found that ranolazine did not reduce the composite rate of ischemia-driven ...
October 22, 2015 — Results from the LEADERS FREE trial found that a polymer-free drug-coated stent (DCS) was superior to ...

October 20, 2015 — Results from the BRAVO 3 trial found that bivalirudin did not significantly reduce major bleeding ...
October 19, 2015 — The U.S. Food and Drug Administration (FDA) granted accelerated approval to Praxbind (idarucizumab) ...
October 2, 2015 — AstraZeneca announced that Brilinta (ticagrelor) 60-mg tablets are now available in U.S. pharmacies ...
September 25, 2015 – The Icahn School of Medicine at Mount Sinai has launched the international TWILIGHT clinical trial ...
September 15, 2015 — AstraZeneca announced that the U.S. Food and Drug Administration (FDA) has approved Brilinta ...
September 11, 2015 — New Haven Pharmaceuticals Inc. announced U.S. Food and Drug Administration (FDA) approval of ...

 December 17, 2015
December 17, 2015