July 16, 2025 — Medtronic has announced that the first patient has been enrolled in the PEripheral Onyx Liquid Embolic (PELE) clinical trial, which will evaluate the safety and effectiveness of the ...
Pulmonary Embolism
This channel includes news and new technology innovations for Pulmonary Embolism. This condition occurs when an artery in the lungs is blocked, usually caused by a blood clot.
Oct. 26, 2025 – Jupiter Endovascular, Inc., a medical technology company developing a new class of endovascular ...
Sept. 17, 2025 — Imperative Care recently announced positive efficacy and safety results from the pivotal Symphony-PE ...
Sept. 9, 2025 — The American Venous Forum (AVF), International Society on Thrombosis and Haemostasis (ISTH), National ...
July 16, 2025 — Medtronic has announced that the first patient has been enrolled in the PEripheral Onyx Liquid Embolic ...
Aug. 29, 2024 – Jupiter Endovascular, Inc., recently announced that the U.S. Food and Drug Administration (FDA) has ...
July 10, 2024 — CellProthera, a private company specializing in cell-based therapies for repairing ischemic tissues, and ...
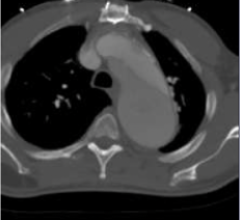
May 7, 2024 — Women and black patients were less frequently treated with minimally invasive therapy compared to men or ...
January 29, 2024 — In patients who undergo pulmonary resection for lung cancer, a major potential postoperative ...
November 2, 2023 — The latest STRIKE-PE data, presented by Penumbra, Inc. at the Vascular Interventional Advances ...
October 31, 2023 — Results of four late-breaking clinical trials presented during the The VEINS Conference 2023, taking ...
October 25, 2023 — Data from the REAL-PE study was presented today at Transcatheter Cardiovascular Therapeutics(TCT) ...
September 27, 2023 — The National PERT Consortium held its 9th Annual Pulmonary Embolism Symposium: “What is Known and ...
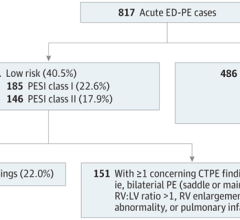
June 30, 2023 — Of approximately 250,000 Americans diagnosed with acute pulmonary embolism, or PE, in emergency ...



 October 27, 2025
October 27, 2025