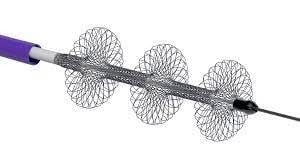
The FlowTriever system
February 16, 2022 – Inari Medical, Inc. has announced that the first patient has been enrolled in PEERLESS, a prospective, randomized controlled trial comparing the outcomes of patients with intermediate-high risk pulmonary embolism (PE) treated with the FlowTriever system versus catheter-directed thrombolysis (CDT).
The first PEERLESS patient was enrolled by Dr. Amir Kaki, Interventional Cardiologist, Ascension St. John Hospital in Michigan. PEERLESS will randomize 550 patients and will also enroll up to 150 patients in a registry cohort for patients who cannot be randomized due to an absolute contraindication to thrombolytics. The trial will include up to 60 centers in the United States and Europe.
“We are excited and honored to enroll the first patient in this landmark clinical trial,” said Kaki. “From our own experience, the FlowTriever system has the potential to change the way we treat PE patients, safely removing significant clot burden while avoiding thrombolytics and procedure-related ICU stay. Ascension St. John’s research team continues to be on the cutting-edge of medical device research and we look forward to contributing and developing the evidence base for the treatment of pulmonary embolism.”
“The start of PEERLESS represents an exciting milestone in the advancement of PE treatment, where randomized clinical data evaluating relevant patient outcomes has been limited,” said Global Co-Principal Investigator, Dr. Carin Gonsalves, Professor of Radiology and Co-Director of the Division of Interventional Radiology at Thomas Jefferson University in Philadelphia, PA. “PEERLESS marks an opportunity to directly compare FlowTriever outcomes to CDT outcomes, addressing current gaps in our understanding of PE and providing critical information to clinicians on the optimal treatment for these patients.”
“The treatment of PE is undergoing a transformation. We have been thrilled by the desire of physicians to move this field forward and generate high-quality data through PEERLESS,” said Dr. Thomas Tu, Inari’s Chief Medical Officer. “We are grateful for the collaboration and dedication of our clinical trial investigators on this first of many PE studies to come.”
For more information: https://www.inarimedical.com/

 October 27, 2025
October 27, 2025