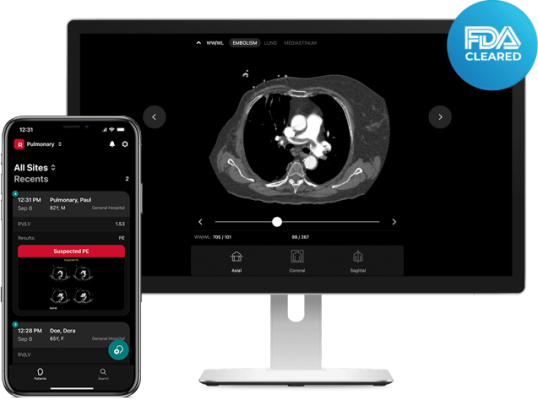
February 22, 2023 — RapidAI, the global leader in neurovascular and vascular AI-enhanced clinical decision support and patient workflow, today announced it has received FDA 510(k) clearance for Rapid RV/LV, the newest addition to the Rapid PE solution. The tool empowers physicians to quickly assess the ratio between the right ventricle (RV) and the left ventricle (LV), a key indicator of pulmonary embolism (PE) severity. Automating this process will enable care teams to more quickly prioritize patients and accelerate decision making.
Rapid RV/LV uses AI to analyze Computerized Tomography Pulmonary Angiograms (CTPAs) and automatically calculate the RV/LV ratio within minutes of the scan, enabling physicians to more quickly conduct risk stratification for patients with right heart strain. Combined with the results from the Rapid PE triage and notification product, which notifies clinicians of suspected PE, and the workflow and communication tools of the Rapid Workflow mobile and web apps, PE care teams now have the information they need at their fingertips to make faster care decisions and to communicate with and activate the team for truly coordinated care.
“Rapid PE streamlines the care pathway from the moment a suspected PE patient is scanned, to diagnosis and through treatment – reducing the complexities that come with managing PE, helping teams triage patients faster, and reducing overall time to treatment,” said Karim Karti, CEO of RapidAI. “The FDA clearance of Rapid RV/LV further enhances our PE solution by providing physicians with an immediate view into patients suffering from RV strain, which is critical to getting the right patients to the right care as fast as possible. This is yet another step toward delivering AI-based solutions that help physicians further enhance patient care and impact patient outcomes to ultimately improve quality of life – something we are very proud to be part of.”
As a component of the Rapid PE solution, the key benefits of Rapid RV/LV include:
- Quickly identifies patients with possible right heart strain
- Reduces time to diagnosis
- Reduces variability and standardizes RV/LV measurement
“Elevated risk PE is a treatable disease, but it is underdiagnosed. Rapid RV/LV will be key in identifying PE patients with right heart strain,” said Dr. Peter Monteleone, Interventional Cardiologist at Ascension Texas Cardiovascular. “The ability to access the RV/LV ratio along with CTPA images will allow clinicians to quickly identify patients who would benefit from further treatment, improving patient outcomes and saving lives. This will have a profound impact on our PE program here at Ascension.”
For more information: https://www.rapidai.com/pe


 September 24, 2025
September 24, 2025