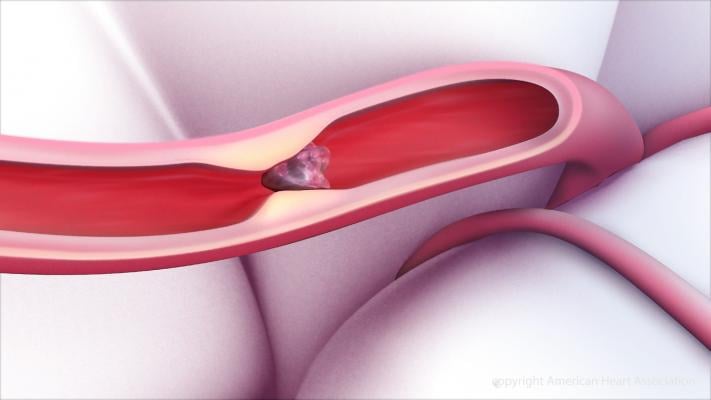
September 7, 2018 — A new Phase 2 clinical trial looks to confirm the efficacy and safety of Thrombolytic Science LLC’s (TSI) sequential dual-treatment regimen of low-dose tissue plasminogen activator (tPA) and HisproUK (TSI’s mutant prourokinase [proUK]) in ischemic stroke patients. The study follows a recently completed Phase 1 study that confirmed the safety and tolerability of TSI’s regimen in healthy volunteers (n=26).
The Phase 2 study will be led by Prof. Diederik Dippel, M.D., Ph.D., co-director of the Erasmus MC Stroke Center in Rotterdam, The Netherlands. It will compare the TSI regimen against high-dose tPA monotherapy – the current standard of care – following an ischemic stroke in patients with distal blood clots (i.e., those not retrievable with a surgical device). TSI also plans to initiate a Phase 2 study of a pre-hospital dual-treatment regimen in heart attack patients, in 2019, for which the company is currently raising funds.
TSI’s flagship product, HisproUK, is a more stable and safer version of proUK. Created by recombinant technology, HisproUK is designed to modulate the high intrinsic activity of proUK, which led to bleeding in previous studies. TSI’s sequential dual-therapy regimen starts with low-dose tPA, which initiates the natural process of thrombolysis. Low dose tPA activates plasminogen into plasmin, which degrades the surface of the clot. HisproUK then activates two additional plasminogen binding sites on the degraded clot surface, a step that accelerates thrombolysis. Together, these natural and synergistic mechanisms enable the process of clot dissolution at low doses.
“Timely and rapid clot dissolution is key to treating ischemic stroke, especially when the clots are distal, a situation that asks for medical treatment with a next generation thrombolytic drug to minimize complications and maximize chances of good outcome,“ explained Dippel. “That makes this Phase 2 trial particularly important, as we hope to show that sequential dual-therapy with low-dose tPA and HisproUK can initiate thrombolysis safely and effectively.”
For more information: www.tsillc.net


 August 28, 2023
August 28, 2023 









