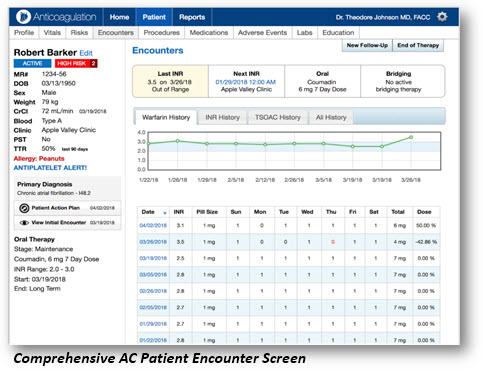
Image courtesy of Point of Care Decision Support
April 30, 2018 — Point of Care Decision Support (PCDS) announced the release of a new version 2.0 of their anticoagulation clinical decision support (CDS) software. The application was developed with leading clinical thrombosis experts to specifically address the new and complex challenges of managing both warfarin and non-vitamin K antagonist oral anticoagulants (NOACs). It enables healthcare provider teams to deliver consistent, measurable and evidence-based quality of care for patients on anticoagulants.
Key benefits to healthcare practitioners that electronically document care for patients on an oral anticoagulant therapy include:
- Central patient dashboard that allows them to understand the patient's history and plan in less than 60 seconds;
- Automatic dosage calculations using validated nomograms to assist in medication management;
- Integration with validated survey instruments such as CHA2DS2-VASc, HAS-BLED and HEMORR2HAGES;
- Full patient plan of care instructions available to print, providing a customized and relevant shared patient/clinician experience;
- Electronic medical record (EMR)-agnostic integration with bidirectional flow of patient data that dramatically decreases data entry; and
- Enhanced reporting options that meet Centers for Medicare and Medicaid Services (CMS)/Medicare Access and CHIP Reauthorization Act (MACRA) "high activity" improvement requirements.
PCDS demonstrated AC 2.0 at the AC Forum Boot Camp for physicians, nurses and pharmacists that work with patients on oral anticoagulant therapies, April 23-24 in Austin, Texas.
For more information: www.ptofcare.com


 August 28, 2023
August 28, 2023 









