February 29, 2024 — The American College of Cardiology (ACC) will soon be holding its ACC 73rd Annual Scientific Session & Expo, ACC.24 from April 6 - 8, 2024 in Atlanta, GA at the Georgia World ...
Clinical Decision Support
This channel includes news and new technology innovations for clinical decision support, CDS, used for diagnostic aids, workflow enhancement, and to select appropriate use criteria (AUC).
May 22, 2025 — Auxira Health has officially launched — the company provides a new approach to clinical support purpose ...
May 28, 2024 — Yoga focused on breathing, meditation, and relaxation is linked with symptom improvement in patients with ...
February 29, 2024 — The American College of Cardiology (ACC) will soon be holding its ACC 73rd Annual Scientific Session ...
February 13, 2024 — Cleerly, a digital healthcare company focused on artificial intelligence (AI)-driven heart disease ...
January 31, 2024 — Boston Scientific Corporation announced it has received U.S. Food and Drug Administration (FDA) appro ...
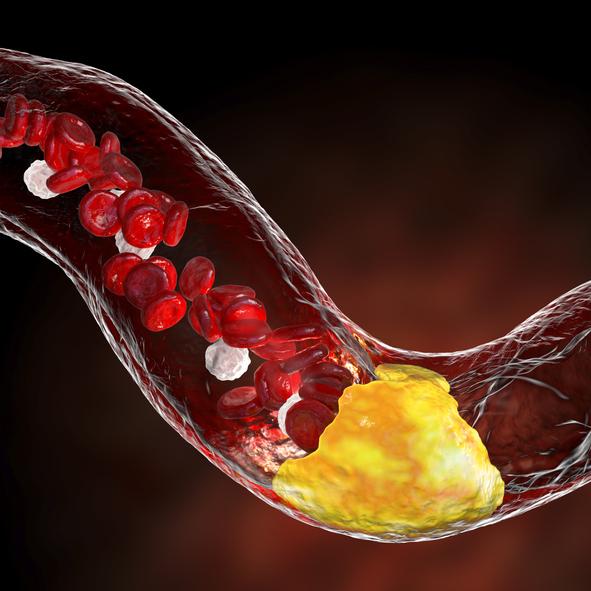
Atherosclerotic cardiovascular disease (ASCVD), caused by plaque buildup in arterial walls, is one of the leading causes ...
January 23, 2024 — Focused on delivering the latest scientific advancements and new treatments in cerebrovascular ...
April 12, 2023 — Results from the Precision Hypertension Care Study (PHYSIC) showed that personalized blood pressure ...
April 11, 2023 — A newly-published study in the Journal of the American College of Cardiology, JACC, the flagship ...
March 21, 2023 — Procyrion, Inc., a medical device company dedicated to improving outcomes for patients with cardiac ...
March 21, 2023 — The first platform to combine real-world cardiology data from millions of people in multiple European ...
January 24, 2023 — Four cardiology organizations have joined forces in a call to action to reinvent randomized clinical ...
December 7, 2022 — Compared with team-based care alone, the addition of a computerized clinical decision support system ...
June 2, 2022 — Elixir Medical, a developer of innovative, drug-eluting cardiovascular devices, announced print ...




 May 23, 2025
May 23, 2025